Abstract
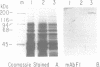
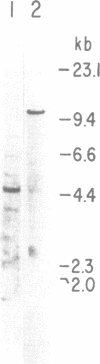
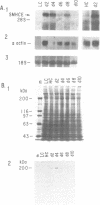
Vertebrate myosin heavy chains (MHC) are represented by multiple genes that are expressed in a spatially and temporally distinct pattern during development. In order to obtain molecular probes for developmentally regulated human MHC isoforms, we used monoclonal antibodies to screen an expression cDNA library constructed from primary human myotube cultures. A 3.4 kb cDNA was isolated that encodes one of the first MHCs to be transcribed in human skeletal muscle development. A portion of the corresponding gene encoding this isoform has also been isolated. Expression of this embryonic MHC is a hallmark of muscle regeneration after birth and is a characteristic marker of human muscular dystrophies. During normal human development, expression is restricted to the embryonic period of development prior to birth. In primary human muscle cell cultures, devoid of other cell types, mRNA accumulation begins as myotubes form, reaches a peak 2 days later and declines to undetectable levels within 10 days. The expression of the protein encoded by the embryonic skeletal MHC gene follows a similar time course, lagging behind the mRNA by approximately two days. Thus, expression of the human embryonic gene is efficiently induced and then repressed in cultured muscle cells, as it is in muscle tissue. The study of the regulation of a human MHC isoform with a central role in muscle development and in muscle regeneration in disease states is therefore amendable to analysis at a molecular level.
Full text
PDF




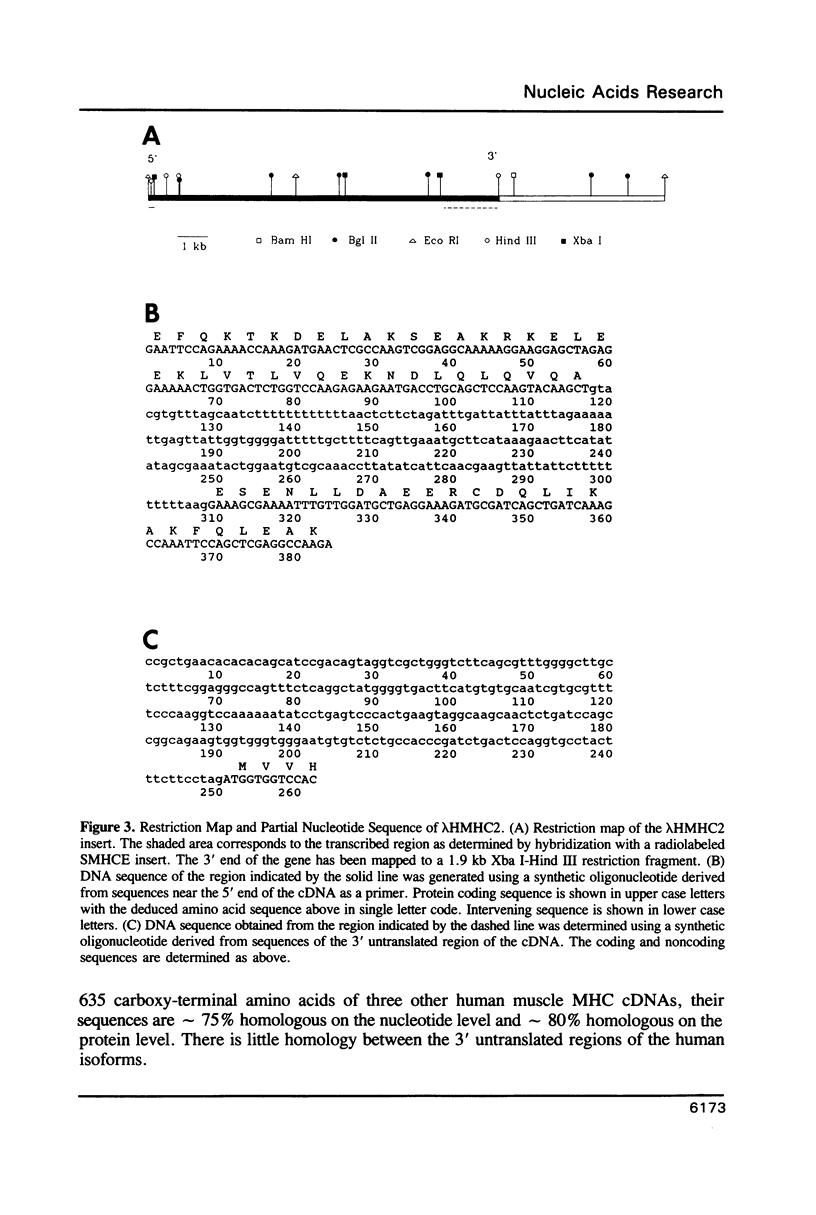


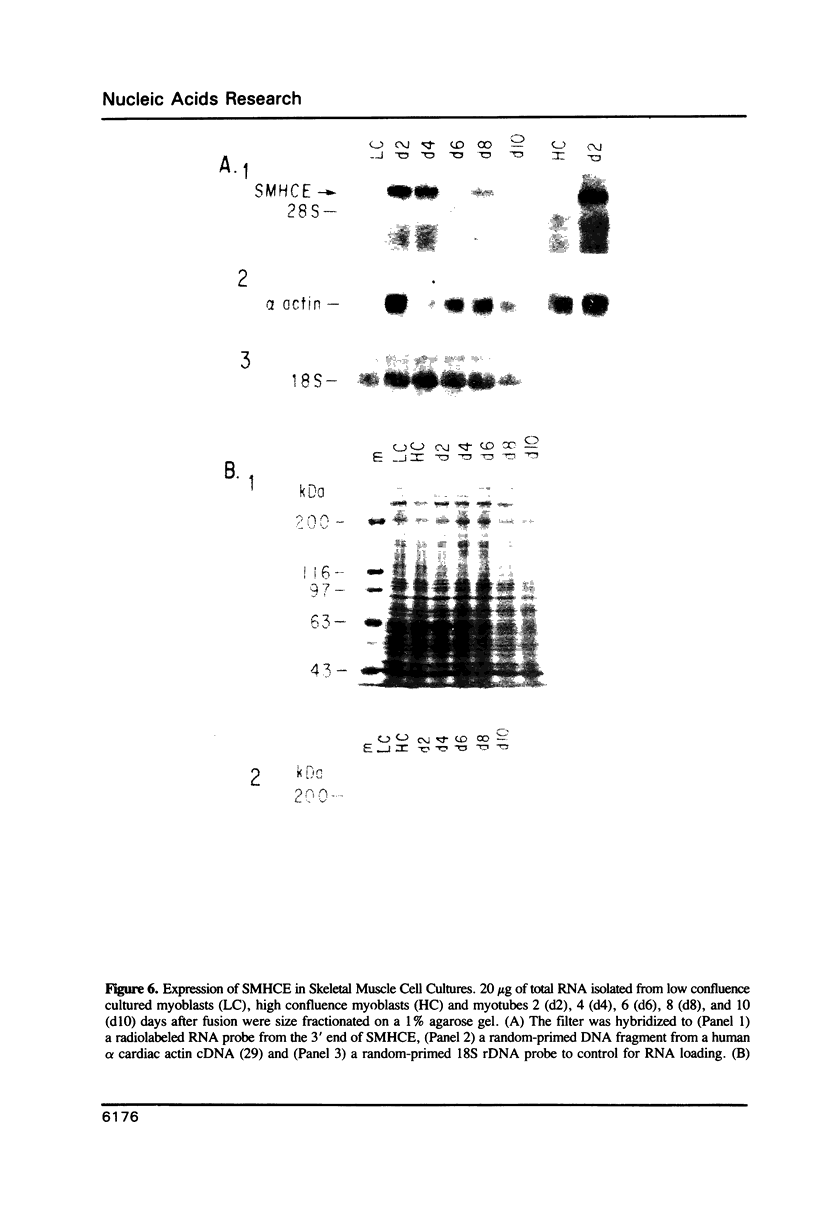



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnheim N., Krystal M., Schmickel R., Wilson G., Ryder O., Zimmer E. Molecular evidence for genetic exchanges among ribosomal genes on nonhomologous chromosomes in man and apes. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7323–7327. doi: 10.1073/pnas.77.12.7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandman E., Matsuda R., Strohman R. C. Developmental appearance of myosin heavy and light chain isoforms in vivo and in vitro in chicken skeletal muscle. Dev Biol. 1982 Oct;93(2):508–518. doi: 10.1016/0012-1606(82)90138-5. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Webster C. Isolation and characterization of human muscle cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5623–5627. doi: 10.1073/pnas.78.9.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerny L. C., Bandman E. Contractile activity is required for the expression of neonatal myosin heavy chain in embryonic chick pectoral muscle cultures. J Cell Biol. 1986 Dec;103(6 Pt 1):2153–2161. doi: 10.1083/jcb.103.6.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs G., Maxson R., Kedes L. H. Histone gene expression during sea urchin embryogenesis: isolation and characterization of early and late messenger RNAs of Strongylocentrotus purpuratus by gene-specific hybridization and template activity. Dev Biol. 1979 Nov;73(1):153–173. doi: 10.1016/0012-1606(79)90144-1. [DOI] [PubMed] [Google Scholar]
- Emerson C. P., Jr, Bernstein S. I. Molecular genetics of myosin. Annu Rev Biochem. 1987;56:695–726. doi: 10.1146/annurev.bi.56.070187.003403. [DOI] [PubMed] [Google Scholar]
- Feghali R., Leinwand L. A. Molecular genetic characterization of a developmentally regulated human perinatal myosin heavy chain. J Cell Biol. 1989 May;108(5):1791–1797. doi: 10.1083/jcb.108.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons R. B., Hoh J. F. Embryonic and foetal myosins in human skeletal muscle. The presence of foetal myosins in duchenne muscular dystrophy and infantile spinal muscular atrophy. J Neurol Sci. 1981 Nov-Dec;52(2-3):367–384. doi: 10.1016/0022-510x(81)90018-6. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Gunning P., Mohun T., Ng S. Y., Ponte P., Kedes L. Evolution of the human sarcomeric-actin genes: evidence for units of selection within the 3' untranslated regions of the mRNAs. J Mol Evol. 1984;20(3-4):202–214. doi: 10.1007/BF02104727. [DOI] [PubMed] [Google Scholar]
- Kraft R., Tardiff J., Krauter K. S., Leinwand L. A. Using mini-prep plasmid DNA for sequencing double stranded templates with Sequenase. Biotechniques. 1988 Jun;6(6):544-6, 549. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leinwand L. A., Saez L., McNally E., Nadal-Ginard B. Isolation and characterization of human myosin heavy chain genes. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3716–3720. doi: 10.1073/pnas.80.12.3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard D. G., Ziff E. B., Greene L. A. Identification and characterization of mRNAs regulated by nerve growth factor in PC12 cells. Mol Cell Biol. 1987 Sep;7(9):3156–3167. doi: 10.1128/mcb.7.9.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- Mahdavi V., Chambers A. P., Nadal-Ginard B. Cardiac alpha- and beta-myosin heavy chain genes are organized in tandem. Proc Natl Acad Sci U S A. 1984 May;81(9):2626–2630. doi: 10.1073/pnas.81.9.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdavi V., Izumo S., Nadal-Ginard B. Developmental and hormonal regulation of sarcomeric myosin heavy chain gene family. Circ Res. 1987 Jun;60(6):804–814. doi: 10.1161/01.res.60.6.804. [DOI] [PubMed] [Google Scholar]
- Mahdavi V., Periasamy M., Nadal-Ginard B. Molecular characterization of two myosin heavy chain genes expressed in the adult heart. Nature. 1982 Jun 24;297(5868):659–664. doi: 10.1038/297659a0. [DOI] [PubMed] [Google Scholar]
- Molina M. I., Kropp K. E., Gulick J., Robbins J. The sequence of an embryonic myosin heavy chain gene and isolation of its corresponding cDNA. J Biol Chem. 1987 May 15;262(14):6478–6488. [PubMed] [Google Scholar]
- Nagai R., Larson D. M., Periasamy M. Characterization of a mammalian smooth muscle myosin heavy chain cDNA clone and its expression in various smooth muscle types. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1047–1051. doi: 10.1073/pnas.85.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILPOTT D. E., SZENT-GYORGYI A. G. The structure of light-meromyosin: an electron microscopic study. Biochim Biophys Acta. 1954 Oct;15(2):165–173. doi: 10.1016/0006-3002(54)90056-6. [DOI] [PubMed] [Google Scholar]
- Periasamy M., Wydro R. M., Strehler-Page M. A., Strehler E. E., Nadal-Ginard B. Characterization of cDNA and genomic sequences corresponding to an embryonic myosin heavy chain. J Biol Chem. 1985 Dec 15;260(29):15856–15862. [PubMed] [Google Scholar]
- Reinach F. C., Fischman D. A. Recombinant DNA approach for defining the primary structure of monoclonal antibody epitopes. The analysis of a conformation-specific antibody to myosin light chain 2. J Mol Biol. 1985 Feb 5;181(3):411–422. doi: 10.1016/0022-2836(85)90229-3. [DOI] [PubMed] [Google Scholar]
- Saez L. J., Gianola K. M., McNally E. M., Feghali R., Eddy R., Shows T. B., Leinwand L. A. Human cardiac myosin heavy chain genes and their linkage in the genome. Nucleic Acids Res. 1987 Jul 10;15(13):5443–5459. doi: 10.1093/nar/15.13.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez L., Leinwand L. A. Characterization of diverse forms of myosin heavy chain expressed in adult human skeletal muscle. Nucleic Acids Res. 1986 Apr 11;14(7):2951–2969. doi: 10.1093/nar/14.7.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S., Gorza L., Sartore S., Saggin L., Carli M. Embryonic myosin heavy chain as a differentiation marker of developing human skeletal muscle and rhabdomyosarcoma. A monoclonal antibody study. Exp Cell Res. 1986 Mar;163(1):211–220. doi: 10.1016/0014-4827(86)90574-4. [DOI] [PubMed] [Google Scholar]
- Silberstein L., Webster S. G., Travis M., Blau H. M. Developmental progression of myosin gene expression in cultured muscle cells. Cell. 1986 Sep 26;46(7):1075–1081. doi: 10.1016/0092-8674(86)90707-5. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strehler E. E., Strehler-Page M. A., Perriard J. C., Periasamy M., Nadal-Ginard B. Complete nucleotide and encoded amino acid sequence of a mammalian myosin heavy chain gene. Evidence against intron-dependent evolution of the rod. J Mol Biol. 1986 Aug 5;190(3):291–317. doi: 10.1016/0022-2836(86)90003-3. [DOI] [PubMed] [Google Scholar]
- Taylor L. D., Bandman E. Distribution of fast myosin heavy chain isoforms in thick filaments of developing chicken pectoral muscle. J Cell Biol. 1989 Feb;108(2):533–542. doi: 10.1083/jcb.108.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrick H. M., Spudich J. A. Myosin structure and function in cell motility. Annu Rev Cell Biol. 1987;3:379–421. doi: 10.1146/annurev.cb.03.110187.002115. [DOI] [PubMed] [Google Scholar]
- Webster C., Pavlath G. K., Parks D. R., Walsh F. S., Blau H. M. Isolation of human myoblasts with the fluorescence-activated cell sorter. Exp Cell Res. 1988 Jan;174(1):252–265. doi: 10.1016/0014-4827(88)90159-0. [DOI] [PubMed] [Google Scholar]
- Webster C., Silberstein L., Hays A. P., Blau H. M. Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell. 1988 Feb 26;52(4):503–513. doi: 10.1016/0092-8674(88)90463-1. [DOI] [PubMed] [Google Scholar]
- Weydert A., Barton P., Harris A. J., Pinset C., Buckingham M. Developmental pattern of mouse skeletal myosin heavy chain gene transcripts in vivo and in vitro. Cell. 1987 Apr 10;49(1):121–129. doi: 10.1016/0092-8674(87)90762-8. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Sell S. M., Butler-Browne G. S., Schwartz K., Bouveret P., Pinset-Härstöm I. Three myosin heavy-chain isozymes appear sequentially in rat muscle development. Nature. 1981 Aug 27;292(5826):805–809. doi: 10.1038/292805a0. [DOI] [PubMed] [Google Scholar]
- Wieczorek D. F., Periasamy M., Butler-Browne G. S., Whalen R. G., Nadal-Ginard B. Co-expression of multiple myosin heavy chain genes, in addition to a tissue-specific one, in extraocular musculature. J Cell Biol. 1985 Aug;101(2):618–629. doi: 10.1083/jcb.101.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]