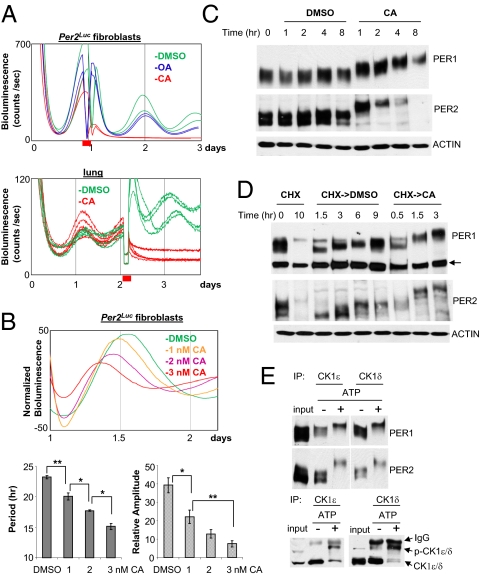
Fig. 3.
Phosphatase inhibition by calyculin A (CA) causes rapid hyperphosphorylation and degradation of PER, and disruption of circadian rhythms in fibroblasts and lung explants. (A) Circadian rhythms are abolished by CA treatment at a high dose. Per2Luc fibroblasts were treated with DMSO, OA, or CA for 2 h at the first peak of the bioluminescence rhythm, then washed and returned to the Lumicycle. Per2Luc lung was treated with DMSO or CA for 2 h near the second peak, then washed and returned to the Lumicycle. Red bar indicates the timing of the treatment. (B) Circadian rhythms are shortened by CA treatment at low doses in a dose-responsive manner. The cells were treated with CA for 6 h at the first peak and then measured for bioluminescence rhythms. Because only one complete cycle was observed after the CA treatment, period and amplitude of the one cycle were compared. (Upper) Comparison of representative traces. (Lower) Period and relative amplitude values presented as mean ± SEM from a representative of several experiments. Sample number: DMSO = 5; 1 nM = 5; 2 nM = 3; 3 nM = 5. P values were calculated by Student t test between two groups. *P < 0.05; **P < 0.01. (C) Hyperphosphorylation and rapid degradation of PER1/2 by CA treatment. Desynchronized Per2Luc fibroblasts were treated with DMSO or CA and harvested at the indicated times. (D) Accelerated PER phosphorylation by CA treatment. Fibroblasts were treated with cycloheximide for 10 h to remove existing PER, washed, and treated with DMSO or CA. The arrow indicates a nonspecific band. (E) In vitro kinase assay of in vivo PER:CK1δ/ε complexes. In vivo PER:CK1δ or ε complexes were purified by immunoprecipitation with antibody to CK1δ or CK1ε, and subjected to in vitro kinase reaction for 30 min. The resulting samples were subjected to immunoblotting for PER1/2 and CK1δ/ε. Note that CK1δ/ε were also phosphorylated by the kinase reaction (p-CK1δ/ε).