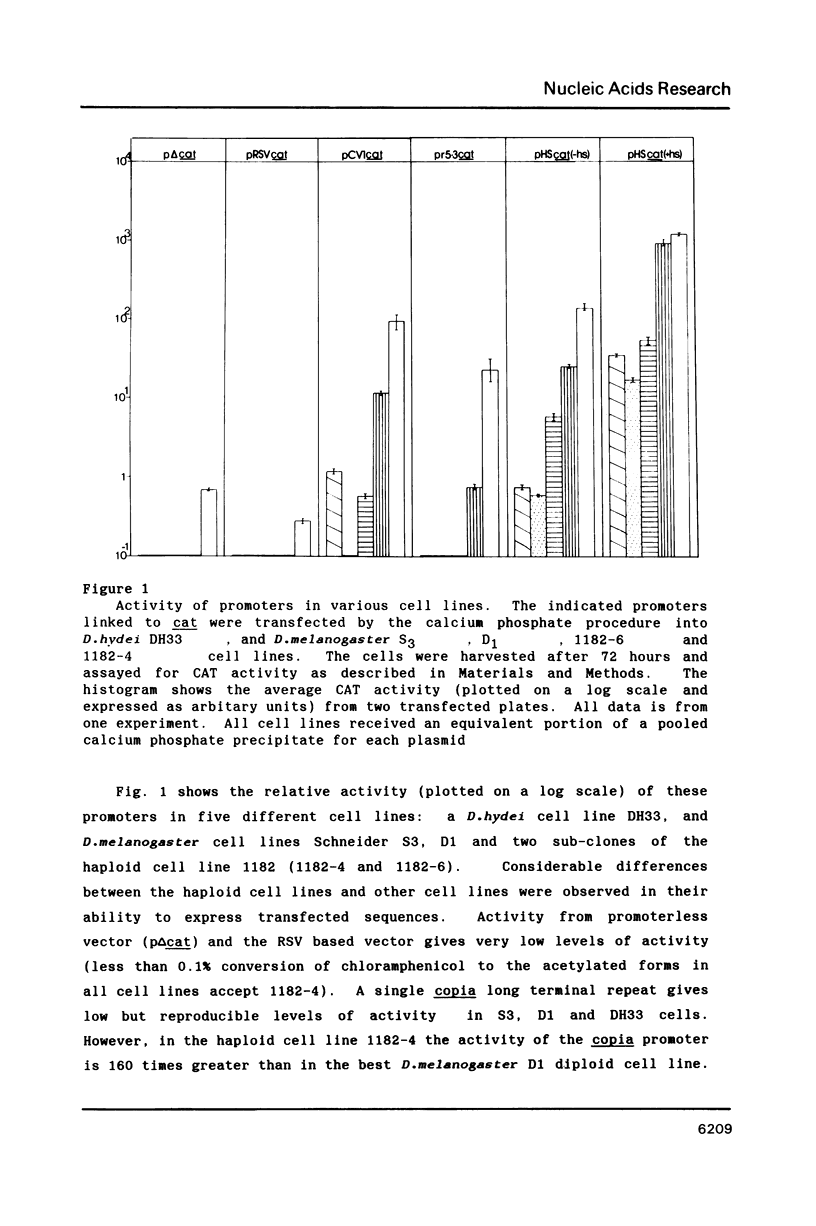
Abstract
Drosophila tissue culture cells have been important in the study of homologous promoters and more recently in the study of mammalian transcriptional factors such as CTF and SP1 which bind and stimulate transcription from transfected genes. In this paper we show that a Drosophila melanogaster haploid cell line (1182-4), not previously used for transfection studies, is capable of taking up and expressing DNA without the use of a facilitating agent such as calcium phosphate. Furthermore expression from a variety of Drosophila promoters such as copia, heatshock and rudimentary as well as a mammalian promoter RSV-LTR, show between 20 and over 100 times more activity in 1182-4 cells than in D.hydei DH33 or D.melanogaster S3, or D1 cell lines. This cell line should prove to be particularly useful for the analysis of weak promoters and heterologous transcription factors.
Full text
PDF




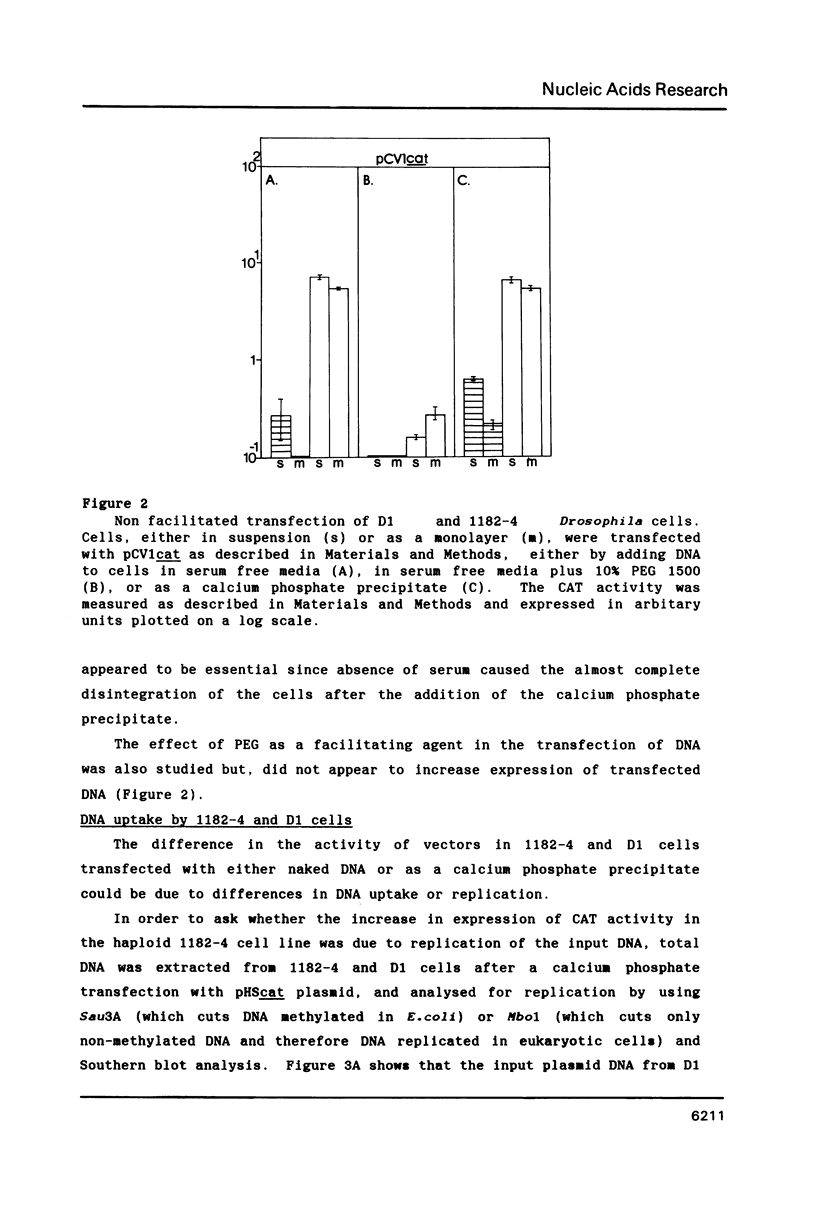
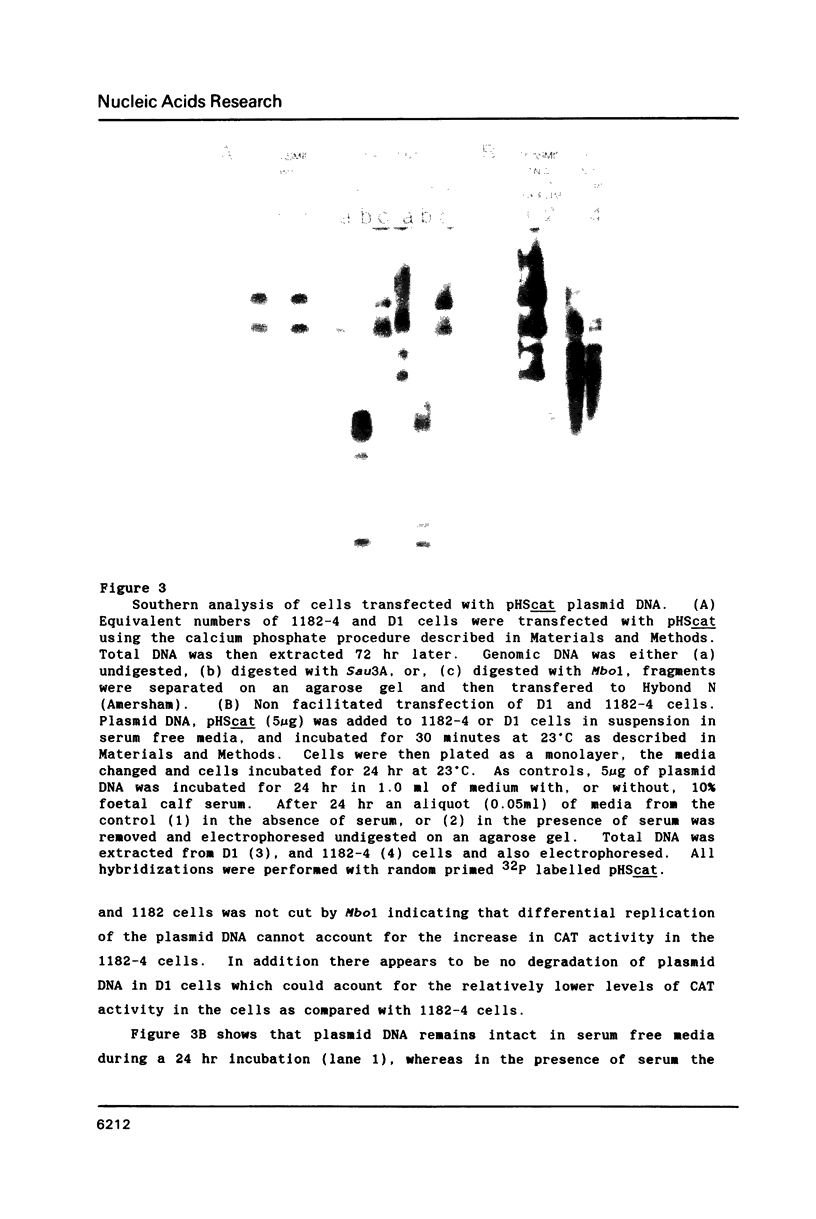




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton N. K., Vapnek D. Nucleotide sequence analysis of the chloramphenicol resistance transposon Tn9. Nature. 1979 Dec 20;282(5741):864–869. doi: 10.1038/282864a0. [DOI] [PubMed] [Google Scholar]
- Burke J. F., Mogg A. E. Construction of a vector, pRSVcatamb38, for the rapid and sensitive assay of amber suppression in human and other mammalian cells. Nucleic Acids Res. 1985 Feb 25;13(4):1317–1326. doi: 10.1093/nar/13.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. F., Sinclair J. H., Sang J. H., Ish-Horowicz D. An assay for transient gene expression in transfected Drosophila cells, using [3H]guanine incorporation. EMBO J. 1984 Nov;3(11):2549–2554. doi: 10.1002/j.1460-2075.1984.tb02172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courey A. J., Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell. 1988 Dec 2;55(5):887–898. doi: 10.1016/0092-8674(88)90144-4. [DOI] [PubMed] [Google Scholar]
- Crabb D. W., Dixon J. E. A method for increasing the sensitivity of chloramphenicol acetyltransferase assays in extracts of transfected cultured cells. Anal Biochem. 1987 May 15;163(1):88–92. doi: 10.1016/0003-2697(87)90096-0. [DOI] [PubMed] [Google Scholar]
- Debec A. Haploid cell cultures of Drosophila melanogaster. Nature. 1978 Jul 20;274(5668):255–256. doi: 10.1038/274255a0. [DOI] [PubMed] [Google Scholar]
- Di Nocera P. P., Dawid I. B. Transient expression of genes introduced into cultured cells of Drosophila. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7095–7098. doi: 10.1073/pnas.80.23.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dübel S., Little M. Microtubule-dependent cell cycle regulation is implicated in the G2 phase of Hydra cells. J Cell Sci. 1988 Nov;91(Pt 3):347–359. doi: 10.1242/jcs.91.3.347. [DOI] [PubMed] [Google Scholar]
- Fountain J. W., Lockwood W. K., Collins F. S. Transfection of primary human skin fibroblasts by electroporation. Gene. 1988 Aug 15;68(1):167–172. doi: 10.1016/0378-1119(88)90610-5. [DOI] [PubMed] [Google Scholar]
- Freund J. N., Zerges W., Schedl P., Jarry B. P., Vergis W. Molecular organization of the rudimentary gene of Drosophila melanogaster. J Mol Biol. 1986 May 5;189(1):25–36. doi: 10.1016/0022-2836(86)90378-5. [DOI] [PubMed] [Google Scholar]
- Frost E., Williams J. Mapping temperature-sensitive and host-range mutations of adenovirus type 5 by marker rescue. Virology. 1978 Nov;91(1):39–50. doi: 10.1016/0042-6822(78)90353-7. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Howard B. H., Reeves R. Expression of recombinant plasmids in mammalian cells is enhanced by sodium butyrate. Nucleic Acids Res. 1983 Nov 11;11(21):7631–7648. doi: 10.1093/nar/11.21.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C., Padmanabhan R., Howard B. H. High efficiency DNA-mediated transformation of primate cells. Science. 1983 Aug 5;221(4610):551–553. doi: 10.1126/science.6306768. [DOI] [PubMed] [Google Scholar]
- Loyter A., Scangos G. A., Ruddle F. H. Mechanisms of DNA uptake by mammalian cells: fate of exogenously added DNA monitored by the use of fluorescent dyes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):422–426. doi: 10.1073/pnas.79.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine R., Levy D. E., Reich N., Darnell J. E., Jr Transcriptional stimulation by CaPO4-DNA precipitates. Nucleic Acids Res. 1988 Feb 25;16(4):1371–1378. doi: 10.1093/nar/16.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls J. M., Freund J. N., Jarry B. P., Louis C., Segraves W. A., Schedl P. Organization of transcription units around the Drosophila melanogaster rudimentary locus and temporal pattern of expression. Mol Gen Genet. 1986 Mar;202(3):493–499. doi: 10.1007/BF00333283. [DOI] [PubMed] [Google Scholar]
- Santoro C., Mermod N., Andrews P. C., Tjian R. A family of human CCAAT-box-binding proteins active in transcription and DNA replication: cloning and expression of multiple cDNAs. Nature. 1988 Jul 21;334(6179):218–224. doi: 10.1038/334218a0. [DOI] [PubMed] [Google Scholar]
- Shen Y. M., Hirschhorn R. R., Mercer W. E., Surmacz E., Tsutsui Y., Soprano K. J., Baserga R. Gene transfer: DNA microinjection compared with DNA transfection with a very high efficiency. Mol Cell Biol. 1982 Sep;2(9):1145–1154. doi: 10.1128/mcb.2.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair J. H., Bryant L. A. 20-Hydroxyecdysone increases levels of transient gene expression in transfected Drosophila cells. Nucleic Acids Res. 1987 Nov 25;15(22):9255–9261. doi: 10.1093/nar/15.22.9255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair J. H., Burke J. F., Ish-Horowicz D., Sang J. H. Functional analysis of the transcriptional control regions of the copia transposable element. EMBO J. 1986 Sep;5(9):2349–2354. doi: 10.1002/j.1460-2075.1986.tb04503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair J. H., Saunders S. E., Burke J. F., Sang J. H. Regulated expression of a Drosophila melanogaster heat shock locus after stable integration in a Drosophila hydei cell line. Mol Cell Biol. 1985 Nov;5(11):3208–3213. doi: 10.1128/mcb.5.11.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompayrac L. M., Danna K. J. Efficient infection of monkey cells with DNA of simian virus 40. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7575–7578. doi: 10.1073/pnas.78.12.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B., Alvarez C. M., Bohman R., O'Connor J. D. An ecdysteroid-induced alteration in the cell cycle of cultured Drosophila cells. Cell. 1980 Dec;22(3):675–682. doi: 10.1016/0092-8674(80)90543-7. [DOI] [PubMed] [Google Scholar]
- Stow N. D., Wilkie N. M. An improved technique for obtaining enhanced infectivity with herpes simplex virus type 1 DNA. J Gen Virol. 1976 Dec;33(3):447–458. doi: 10.1099/0022-1317-33-3-447. [DOI] [PubMed] [Google Scholar]
- Wu C. Two protein-binding sites in chromatin implicated in the activation of heat-shock genes. Nature. 1984 May 17;309(5965):229–234. doi: 10.1038/309229a0. [DOI] [PubMed] [Google Scholar]