Abstract
Introduction
GTS-21 ((3E)-3-[(2,4-dimethoxyphenyl)methylene]-3,4,5,6-tetrahydro-2,3′-bipyridine), a partial α7 nicotinic acetylcholine receptor agonist drug, has recently been shown to improve cognition in schizophrenia and Alzheimer’s disease. One of its two major demethylated metabolites, 4-OH-GTS-21, has been suggested to contribute to its therapeutic effects.
Methods
We labeled GTS-21 in two different positions with carbon-11 ([2-methoxy-11C]GTS-21 and [4-methoxy-11C]GTS-21) along with two corresponding demethylated metabolites ([2-methoxy-11C]4-OH-GTS-21 and [4-methoxy-11C]2-OH-GTS-21) for pharmacokinetic studies in baboons and mice with PET.
Results
Both [2-methoxy-11C]GTS-21 and [4-methoxy-11C]GTS-21 showed similar initial high rapid uptake in baboon brain, peaking from 1–3.5 min (0.027–0.038 %ID/cc) followed by rapid clearance (t1/2 <15 min), resulting in low brain retention by 30 min. However, after 30 min, [2-methoxy-11C]GTS-21 continued to clear while [4-methoxy-11C]GTS-21 plateaued, suggesting the entry of a labeled metabolite into the brain. Comparison of the pharmacokinetics of the two labeled metabolites confirmed expected higher brain uptake and retention of [4-methoxy-11C]2-OH-GTS-21 (the labeled metabolite of [4-methoxy-11C]GTS-21) relative to [2-methoxy-11C]4-OH-GTS-21 (the labeled metabolite of [2-methoxy-11C]GTS-21) which had negligible brain uptake. Ex vivo studies in mice showed that GTS-21 is the major chemical form in the mouse brain. Whole body dynamic PET imaging in baboon and mouse showed that the major route of excretion of C-11 is through the gallbladder.
Conclusions
The major findings are (1) extremely rapid uptake and clearance of [2-methoxy-11C]GTS-21 from the brain which may need to be considered in developing optimal dosing of GTS-21 for patients, and (2) significant brain uptake of 2-OH-GTS-21, suggesting that it might contribute to the therapeutic effects of GTS-21. This study illustrates the value of comparing different label positions and labeled metabolites to gain insight on the behavior of a CNS drug and its metabolites in the brain providing an important perspective on drug pharmacokinetics.
Keywords: [11C]GTS-21, Carbon-11, α7 nicotinic acetylcholine receptor, drug pharmacokinetics, PET, labeled metabolites
1. Introduction
The neuronal nicotinic acetylcholine receptor (nAChR) in the central nervous system (CNS) is an important molecular target for the development of drugs for the treatment of Alzheimer’s disease (AD), schizophrenia and tobacco dependence [1,2]. Drug development in this area is complicated by the fact that alterations of nAChR in neurodegenerative diseases are receptor subtype-specific [3]. In the brain, α4β2 and α7 nAChR subtypes predominate. These have different distribution patterns in different brain regions [1]. Over the last decade considerable effort has been dedicated to the development of radiotracers that can be used to selectively and quantitatively examine the distribution of nAChR subtypes in the human brain with positron emission tomography (PET) and single photon emission computed tomography (SPECT). This effort has been rewarded by the development of a number of good radiotracers for the α4β2 nAChR subtype [4]. However, though efforts are underway to develop radiotracers for selective imaging of the α7 subtype, this has been much more challenging due to the lack of lead structures with high affinity and with functional groups that can be labeled with PET and SPECT isotopes [5].
The α7 nAChR subtype is of particular interest in drug research and development because it is involved in the modulation and release of other neurotransmitters such as γ-aminobutyric acid (GABA) and glutamate, and in the activation of various down-stream signaling pathways [6]. Pharmacologically, α7 nAChR agonist activity is associated with neuroprotection, as well as enhancement in cognition and memory [7–11]. This rich pharmacological activity has stimulated the development of nAChR drugs with agonist activity at the α7 nAChR receptor.
One of the most promising α7 nAChR agonist drugs currently in clinical trials is GTS-21 (2, also called DMXB) (Fig. 1) [12–14]. It is a partial agonist for α7 nAChR (Ki= 211 nM against [125I] α-bungarotoxin in rat brain membrane) [15]. It also binds to the α4β2 nAChR (Ki= 84 nM against [3H]cytisine in rat brain membrane) and 5-HT3A receptor (Ki= 0.53 ± 0.9 μmol against [3H]GR65630 in membrane of NCB-20 cells) [15,16]. GTS-21 was first synthesized in 1993 from anabaseine (1, Fig. 1), a natural product isolated from a marine worm nemertines and the Aphaenogaster ant [17,18]. Many preclinical studies have documented the neuroprotective properties of GTS-21 and its metabolites, which included the reduction of Aβ protein- and ethanol-induced neurotoxicity and the enhancement of cell survival [9,19]. Preclinical studies also supported its efficacy in enhancing cognition and learning [20–24] and set the stage for clinical trials in Alzheimer’s disease [13]. Reports that the α7 nAChR system is intact in the cortex in AD brain, whereas α4β2 nAChR is not, further supported translation of GTS-21 to humans [25,26]. In a phase I trial for AD, GTS-21 enhanced cognition in a dose dependent manner without the side effects seen with nicotine itself [13]. Because of its ability to normalize auditory gating deficit in a rodent model, GTS-21 is also under investigation for the treatment of schizophrenia where it was recently reported to enhance cognition in patients with schizophrenia [14,27]. However, the utility of GTS-21 as a drug has been challenged by Tatsumi et al. who comments that it “fails to show a satisfactory pharmacokinetics profiles (PK) in the areas of bioavailability and brain permeability” [28].
Fig. 1.
The structures of anabaseine (1) and GTS-21 (2).
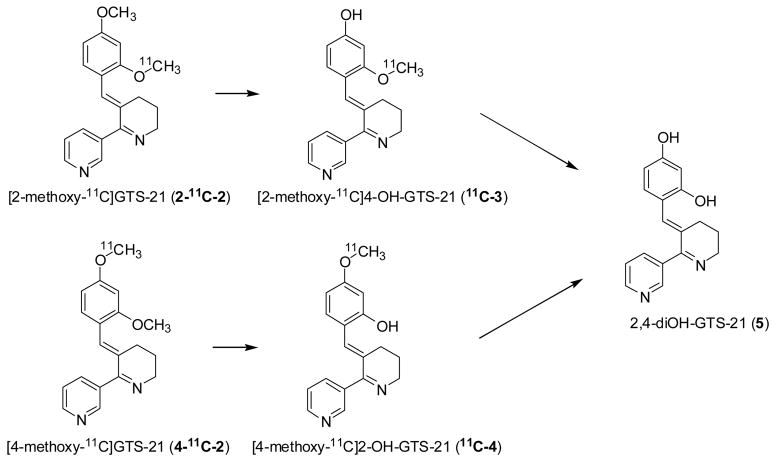
The primary route of metabolism of GTS-21 is O-demethylation. There are three metabolites: 4-OH-GTS-21 (3), 2-OH-GTS-21 (4), and 2,4-diOH-GTS-21 (5) (Fig. 2) [13,29]. Interestingly, one of the major metabolites, 4-OH-GTS-21 (3, EC50= 4.0 μM) is more potent and efficacious than GTS-21 (EC50= 6.0 μM) itself in in vitro functional assay on human α7 nAChR, suggesting that GTS-21 may be a pro-drug for 4-OH-GTS-21 [30]. While 2-OH-GTS-21 (4, EC50= 2.1 μM) is also more efficacious and potent, it has been reported to have poor brain penetrability compared with 4-OH-GTS-21 (3) and GTS-21 in the rat model [31]. However, even though there is evidence that the two metabolites of GTS-21 may be more efficacious at the α7 nAChR than GTS-21 itself, there is no information on the brain pharmacokinetics of either GTS-21 or its demethylated metabolites in primate brain and thus the potential relevance of metabolites to the therapeutic effects of GTS-21 in humans is not known [31].
Fig. 2.
The metabolic pathways of GTS-21 [31] showing the positions of the label for the respective demethylated metabolites from 2-11C-2 and 4-11C-2.
Here, we describe the development and comparison of two regiospecifically labeled [11C]GTS-21 isotopomers (2-11C-2 and 4-11C-2) as tools to measure GTS-21 (2) distribution and pharmacokinetics in brain and peripheral organs in baboon and in mouse using PET. GTS-21 was labeled in two different positions because the profile of labeled metabolites would differ and we reasoned that this would provide insight on bioavailability of labeled metabolite such as 4-OH-GTS-21 (3) in the brain. We also synthesized the C-11 labeled demethylated metabolites of GTS-21, [2-methoxy-11C]4-OH-GTS-21 (11C-3), [4-methoxy-11C]2-OH-GTS-21 (11C-4), to measure their brain penetration and pharmacokinetics. These investigations, in principle, would allow us to better understand the potential contribution of the metabolite to the drug therapeutic effect. In addition, the effect of a therapeutic dose of GTS-21 on the brain pharmacokinetics of 2-11C-2 was assessed. Finally, we performed whole body PET studies in mice with both isotopomers and measured the chemical form of C-11 in the brain and plasma after the administration of 2-11C-2.
2. Materials and methods
2.1. General
Anabaseine was purchased from Toronto Research Chemicals Inc. (North York, Ontario, Canada). GTS-21 (2), 4-OH-GTS-21(3), and 2-OH-GTS-21 (4), 2,4-diOH-GTS-21 (5) were synthesized by known methods [32]. All other chemicals were purchased from Aldrich Chemical Company (Milwaukee, WI, USA) and were used without further purification. NMR spectra were recorded using a Bruker Avance 400 MHz NMR spectrometer (Bruker Instruments Inc. Billerica, MA, USA). During the radiosynthesis, carbon-11 was measured by a Capintec CRC-712MV radioisotope calibrator (Capintec Inc., Ramsey, NJ, USA). [11C]Methyl iodide was synthesized using PETtrace MeI Microlab (GE Medical Systems, Milwaukee, WI, USA) from [11C]carbon dioxide which is produced by an EBCO cyclotron. [11C]Carbon dioxide was generated by the nuclear reaction, 14N(p, α)11C, using a nitrogen/oxygen (1000 ppm) target. Semi-preparative high performance liquid chromatography (HPLC) was performed by a Knauer HPLC system (Sonntek Inc., Woodcliff Lake, NJ, USA) with a model K-5000 pump, a model 87 variable wavelength monitor, and NaI radioactivity detector. Specific activity was determined by measuring the radioactivity and mass; the latter is derived from a standard curve at UV (254 nm) using different concentrations of the authentic reference compounds. Radiochemical purity was also determined by thin layer chromatography (TLC) using and measuring radioactivity distribution on Macherey–Nagel polygram sil G/UV254 plastic-back TLC plate with Bioscan system 200 imaging scanner (Bioscan Inc., Washington, DC). 11C radioactivity was measured by a Packard MINAXI γ5000 automated gamma counter (Packard Instrument, Meriden, CT). All measurements were decay-corrected. All experiments with animals were approved by the Brookhaven Institutional Animal Care and Use Committee.
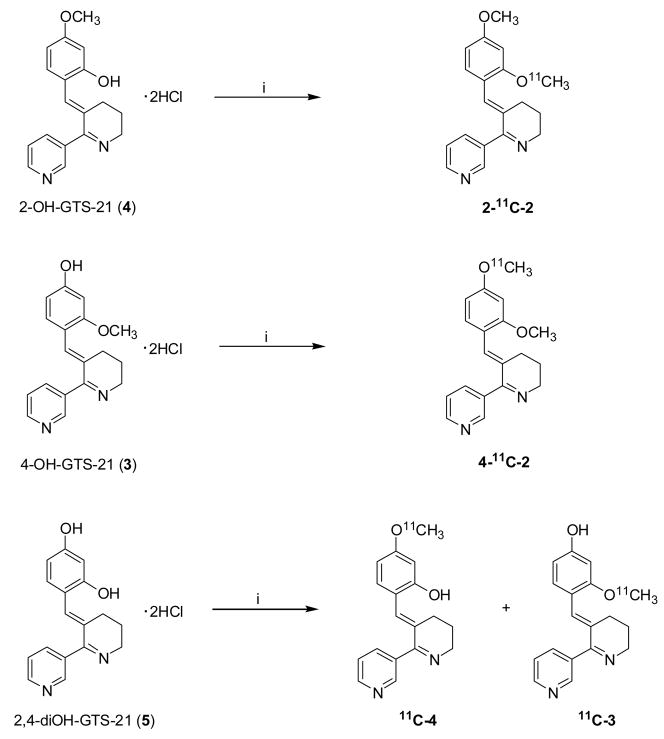
2.2. Synthesis of (3E)-3-[[2-(methoxy-11C)-4-methoxyphenyl]methylene]-3,4,5,6-tetrahydro-2,3′-bipyridine (2-11C-2) and (3E)-3-[[2-methoxy-4-(methoxy-11C)phenyl]methylene]-3,4,5,6-tetrahydro-2,3′-bipyridine (4-11C-2)
A solution of (E)-3-(2-hydroxy-4-methoxybenzylidene)anabaseine (4) dihydrochloride (or (E)-3-(4-hydroxy-2-methoxybenzylidene)anabaseine (3) dihydrochloride) (1 mg, 2.7 μmol) in 0.3 ml of N,N-dimethylformamide and 5.8 μL of NaOH (6 N) was stirred until the color changed to yellow (Fig. 3). The solution was cooled in a dry ice/acetonitrile bath and [11C]methyl iodide was purged into the solution and trapped. When measured 11C peaked, the reaction vessel was sealed and heated at 100 °C for 3 minutes in an oil bath, then cooled for five seconds in a cooling bath (dry ice/acetonitrile). The reaction mixture was diluted with 1 ml of HPLC eluent, then purified with HPLC using water/acetonitrile (60/40) including triethylamine (0.4%(v/v)) at a flow rate 5 ml/min on a semi-preparative Gemini C18 (Phenomenex, 250 mm × 10 mm, 5μ). The product was collected at the expected retention time (RT= 14 min) and solvent was removed by azeotropic evaporation with acetonitrile. After dilution with saline (4 mL, baboon; 2 mL, mouse), the solution was filtered through a Acrodisc® 13 mm Syringe Filter with 0.2 μm HT Tuffryn® Membrane (Pall cooperation, Ann Arbor, MI) into a sterile vial for delivery. For quality control, thin-layer chromatography (TLC) was performed with eluent (chloroform:methanol:ammonia solution= 9:1:0.06); Rf value of 0.7 for carbon-11 which was congruent with an authentic standard of GTS-21 (2) co-spotted with the sample and detected by UV (λ= 254 nm).
Fig. 3.
The radiosynthesis of C-11 labeled GTS-21 and its metabolites. Reagent and condition: i) [11C]CH3I, aqueous sodium hydroxide in DMF, 100 °C, 3 min.
2.3. Synthesis of (3E)-3-[[2-hydroxy-4-(methoxy-11C)phenyl]methylene]-3,4,5,6-tetrahydro-2,3′-bipyridine (11C-4) and (3E)-3-[[4-hydroxy-2-(methoxy-11C)phenyl]methylene]-3,4,5,6-tetrahydro-2,3′-bipyridine (11C-3)
A solution of (E)-3-(2,4-dihydroxybenzylidene)anabaseine (5) dihydrochloride was used as a starting material in the same procedure described in 2.2. 11C-3 was purified with HPLC using 0.1 M ammonium formate solution (water/acetonitrile= 80/20) at a flow rate 5 ml/min on a semi-preparative Gemini C18 (Phenomenex, 250 mm × 10 mm, 5μ), eluting at 18.8 min. The same column and conditions were used for purification of 11C-4 with 0.1 M ammonium formate solution (water/acetonitrile=75/25) and collected at 17 min. Two labeled compounds was analyzed by TLC with eluent (chloroform:methanol:ammonia solution= 8:2:0.4). Rf were 0.77 and 0.74 for 11C-4 and 11C-3, respectively.
2.4. Measurement of free fraction in plasma and log D
A modification of a literature procedure for measurement of the free fraction of [11C]GTS-21 in baboon plasma was used [33]. An aliquot of [11C]GTS-21 was measured for radioactivity and added to 500 μl of baboon plasma, and this mixture was incubated for 10 min at room temperature. Aliquots (20–40 μL) of the incubated spike plasma were assayed for radioactivity. A portion of the incubation mixture (200–400 μL) was placed in the upper level of a Centrifree tube (Amicon Inc, Beverly, MA, USA) and this was centrifuged for 10 minutes. After centrifuging, the top portion of the Centrifree tube containing the bound portion was removed and discarded, and precisely measured aliquots (20–40 μL) of the liquid in the cup (unbound fraction) were counted. The free fraction is the ratio of the decay-corrected counts of the unbound aliquots to the decay-corrected counts of the unspun aliquots.
Log D (an index for lipophilicity) at pH= 7.4 was also measured using a previous method [33,34]. Briefly, an aliquot (50 μL) of [11C]GTS-21 solution was added to a mixture of 1-octanol (2.5 mL) and phosphate buffered saline (PBS, pH 7.4; 2.5 ml). The mixture was vortexed at room temperature for 2 min and then centrifuged at 7000 rpm for 2 min. An aliquot (0.1 mL) of the octanol layer and 1.0 ml of the buffer layer were sampled separately into two empty vials and counted. Two mL of the octanol layer was transferred into a test tube containing 0.5 ml of fresh octanol and 2.5 ml of buffer and the process of vortexing and centrifuging was repeated. The aliquots from each layer were extracted and counted until 6 measures of the ratio of counts in the octanol to counts in the buffer were obtained. Log D is as the log10 of the average of the ratios of the decay corrected counts in the octanol:buffer.
2.5. PET studies in baboon
Four adult female Papio Anubis baboons were anesthetized by an intramuscular injection of ketamine hydrochloride (10 mg/kg), and then maintained with oxygen (800 ml/min), nitrous oxide (1500 ml/min), and isoflurane (Forane, 1–4%) during the scanning. Either 2-11C-2 or 4-11C-2 (2–4 mCi in saline) was injected through a catheter placed in a radial arm vein and arterial blood was sampled through a catheter in popliteal artery with the following time intervals; every 5 sec for 2 min, then 2, 5, 10, 20, 30, 60, 90 min. Heart rate, respiration rate, PO2, and body temperature were checked during the PET scanning. Dynamic PET Imaging was performed by Siemens HR + (Siemen’s high-resolution, whole-body PET scanner with 4.5 ×4.5 × 4.8 mm at center of field of view) for a total of 90 min with the following time frames in 3D mode (10 × 60 sec; 4 × 300 sec; 8 × 450 sec). Either the brain or the torso was placed in the field of view. Prior to the study, a transmission scan was obtained by rotating a 68Ge rod source to correct for attenuation. Each animal was scanned with either 2-11C-2 or 4-11C-2 for the baseline followed by a 2 hour interval after which the other isotopomer was administered (Table 1). To evaluate reproducibility, 2-11C-2 was injected twice with a 2 hr interval between injections. To assess the effect of a therapeutic dose of GTS-21, a baboon was scanned at baseline with 2-11C-2. Two hours later, an intravenous dose of GTS-21 (2) dihydrochloride (0.031 mg/kg; 81.3 nmol/kg based on the literature [21]) was co-administered with 2-11C-2 and the baboon was scanned following the same scanning protocol described above.
Table 1.
Summary of baboon PET studies
| Study # | Baboon | Radiotracer/drug | Brain/Torso |
|---|---|---|---|
| Test-retest in the brain | |||
| BEJ330dy1 | Pearl | 2-11C-2 | brain |
| BEJ330dy2 | Pearl | 2-11C-2 | brain |
|
| |||
| Comparison of two isotopomers of [11C]GTS-21 | |||
| BEJ319 dy1 | April | 2-11C-2 | brain |
| BEJ319 dy2 | April | 4-11C-2 | brain |
| BEH154dy1 | April | 4-11C-2 | brain |
| BEH154dy2 | April | 2-11C-2 | brain |
| BEJ324dy1 | April | 4-11C-2 | brain |
|
| |||
| Comparison of two labeled metabolites | |||
| BEJ335dy1 | Daisy | 11C-4 | brain |
| BEJ335dy2 | Daisy | 11C-3 | brain |
|
| |||
| Co-injection with therapeutic dose of GTS-21 (0.031 mg/kg) | |||
| BEJ323dy1 | Missy | 2-11C-2 | brain |
| BEJ323dy2 | Missy | 2-11C-2/GTS-21 | brain |
|
| |||
| Distribution of peripheral organ | |||
| BEJ327dy1 | Daisy | 2-11C-2 | torso |
To compare the pharmacokinetics of the demethylated GTS metabolites (3 and 4), PET studies in baboons were carried out using 11C-3 and 11C-4 with a 2 hour interval between injections. Arterial plasma samples were obtained over the 90 min time course of the study.
2.5.1 Image Analysis
All image data were reconstructed using filtered back-projection (FBP). For image analysis, we constructed a region of interest (ROI) file with the published MR template and H215O template images [35] by PMOD (PMOD Technologies, Ltd.). The ROI for the global uptake was obtained by selecting four representative transaxial planes. After ECAT7 files were converted to ANALYZE format and all time frames were summed for each file, they were co-registered with H215O template images without normalization. ROI’s were applied to the summed images and adjusted manually for each region and projected onto the dynamic frames to provide time-activity curves for each brain region. Time activity curves were normalized with injected dose to give % injected dose/cc. Model terms K1 and distribution volume (DV) were determined by Logan graphical analysis to evaluate the effect of a therapeutic dose of GTS-21 (2) [36]. ROI’s for peripheral organs were drawn manually in the dynamic images.
2.5.2. Plasma Analysis of [11C]GTS-21 (2-11C-2 and 4-11C-2)
All arterial blood samples were centrifuged and the resulting plasma samples were assayed for radioactivity in a well counter to give the total carbon-11 concentration at each sampling time point. Selected plasma samples (1, 5, 10, 30, 60 min) were further analyzed for unchanged radiotracer using the following procedure. Each sample (0.1–0.4 mL) was added to acetonitrile (0.3 mL), spiked with unlabeled GTS-21 and demethylated metabolites 3 and 4, then sonicated (Polytron, Brinkmann Instruments, Westbury, NY, USA) to disrupt cells. After centrifugation for 3 min, the pellet and supernatant solution were counted and the supernatant was subjected to HPLC analysis (Phenomenex Ultremex 5 C18, 250 mm × 4.60 mm eluting with 100 mM phosphate buffer (pH = 4.5)/methanol (60/40) analysis with UV detector (λ= 254 nm)). The retention time (Rt) of [11C]GTS-21 was 13.8 min at a flow rate of 0.7 mL/min (Rt = 9 min for 11C-3 and 11C-4). Fractions were collected and counted, noting the fractions containing the standards. The ratio of the C-11 in the fraction containing the GTS-21 to the C-11 in the sum of all fractions was used to correct the arterial plasma time-activity curve to give the input function for [11C]GTS-21.
2.5.3. Plasma Analysis of 11C-3 and 11C-4
Arterial plasma samples were analyzed by solid phase extraction [37] after the baboon was injected with the labeled demethylated metabolites 11C-3 and 11C-4. Briefly, the Sep-Pak was conditioned by rinsing with 5 mL of methanol followed by 5 mL of water. A sample of plasma (50–600 μL) was diluted with water (3 mL), then poured onto Bond Elut LRC-C18–500 mg (Varian, Harbor City, CA) pre-loaded with deionized water (2 mL). The combined water fractions (3 mL + 2 mL) were pushed through the Sep-Pak with nitrogen and collected. The Sep-Pak was then rinsed sequentially with water (5 mL × 2) and 50% aqueous methanol (5 mL × 2). Each fraction of eluent was collected separately and counted. Finally, the radioactivity remaining on the Sep-Pak was assayed. The Sep-Pak and the last fraction of eluent contained 11C-3 and 11C-4.
2.6. Mouse Studies
2.6.1. PET study
Four male mice (Swiss-Webster, 30–40 g) were imaged with small animal PET (MicroPET R4™, Siemens) after the injection of 2-11C-2 or 4-11C-2; two were scanned for 20 min and sacrificed for HPLC analysis of brain and plasma and the other two mice were scanned for 40 min to get the whole body distribution and kinetics after injection of 2-11C-2 and 4-11C-2, respectively. Mice were anesthetized using ketamine/xylazine (90/10, 100mg/kg) and positioned prone in the scanner. Radiotracers were administered by intravenous injection in the tail vein (300–438 μCi, specific activity = 1.8–2.9 Ci/μmole at EOB). Data acquisition was started simultaneously with tracer injection and continued for 20–40 min. List mode data was binned into a maximum of 22 frames (5 × 2 sec, 10 × 5 sec, 7 × 300 sec) using microPET Manager (v. 2.3.3.0) software. The resulting sinograms were scatter-corrected using a custom tail-fit method and reconstructed by two dimensional filtered-back projection (FBP) after Fourier rebinning using a filter cutoff at the Nyquist criteria (default parameters of software) [38]. Time-activity curves for the brain, liver, kidney, and gall bladder were determined using ASIPro VM software by drawing regions of interest directly on the summed frame PET image and projected onto the individual dynamic frames.
2.6.2. Ex vivo biodistribution studies (n=3)
Following 20 min or 40 min PET scans with 2-11C-2 or 4-11C-2, two mice were sacrificed and decapitated. Trunk blood, brain, heart, kidney, spleen, lung, liver, gallbladder, and stomach were harvested consecutively. Each organ was weighed and counted to determine the % injected dose/g. Arterial blood was centrifuged for 3 min to give a plasma sample. To determine the ratio of the brain to plasma of GTS-21 and its labeled metabolites, one mouse was sacrificed and decapitated at 20 min after injection of 2-11C-2. The whole brain was homogenized in methanol (1 ml) using a Tissue Tearor (Biospec Producs, Bartlesville, OK) with medium speed for 1 min, centrifuged and the homogenate was filtered to give the supernatant solution. This brain extract was subjected to HPLC analysis using the same conditions which were used to analyze the baboon plasma.
3. Results and Discussion
3.1. Radiolabeling, log D and free fraction in plasma
A moderate radiochemical yield was obtained for both 2-11C-2 and 4-11C-2, ranging from 20–43% corrected to the end of cyclotron bombardment (EOB). Specific activities ranged from 0.8–5.1 Ci/μmol at EOB and radiochemical purities were > 98% for the two labeled compounds. The total synthesis time, from the end of the cyclotron bombardment to delivery for PET studies, was approximately 50 min. The radiochemical yield was not improved by using longer reaction times or higher temperatures. Initial attempts to separate the product from the precursor and side products using 20 mM phosphate buffer (pH = 2.8)/acetonitrile (77.2/22.8) at a flow rate 4 ml/min on a semi-preparative Luna C18 (Phenomenex, 250 mm × 10 mm, 5μ) were not successful. However, when the pH was adjusted to 12 with triethylamine on a Phenomenex Gemini column, a satisfactory and reproducible separation was achieved. Labeled demethylated metabolites, 11C-3 and 11C-4 were synthesized in 12-15% yield with >99% radiochemical purity and specific activity 5.1–6.0 Ci/μmol.
The log D at pH = 7.4 (n= 4) of [11C]GTS-21 was 2.82 which is, in principle, suitable for blood-brain barrier (BBB) penetration [39]. As expected, two labeled metabolites have lower log D (2.10 for 11C-4, 2.07 for 11C-3) than [11C]GTS-21. We note that the free fractions in the plasma were 4.47%, 9.32%, 15.47% for [11C]GTS-21, 11C-4, 11C-3, respectively, which are similar to values reported elsewhere [31].
3.2 Biological studies
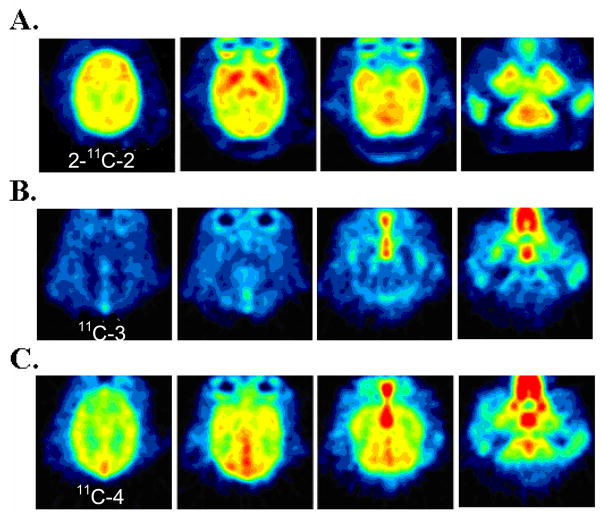
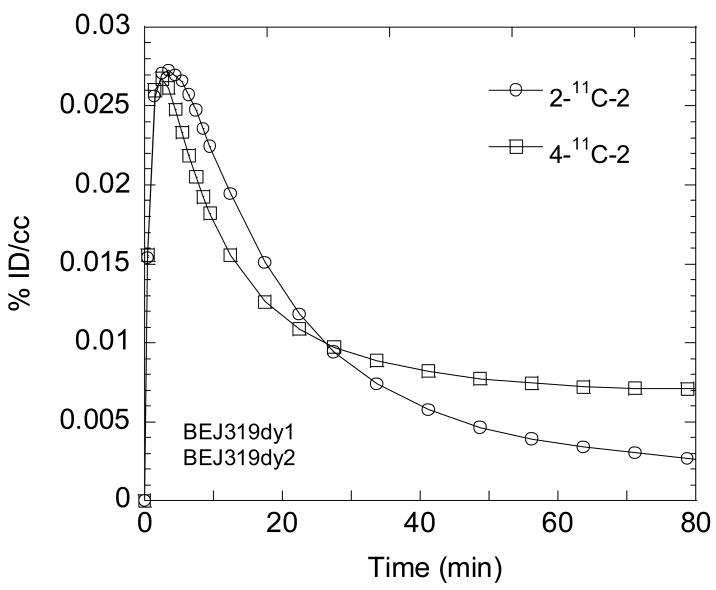
For both 2-11C-2 and 4-11C-2, carbon-11 was widely distributed to cortical and subcortical brain regions (see Fig. 4A for summed images). Brain entry was rapid and uptake was high. For example, in the cerebellum (which is similar to other brain regions), the average peak uptake occurred at 1.4 ± 0.2 and 1.7 ± 0.7 min and the average uptakes at peak were 0.036 ± 0.0038 and 0.037 ± 0.0066 for 2-11C-2 and 4-11C-2 respectively. However, clearance was also very rapid, showing that half time for clearance from peak averaged 9.5 ± 1.9 and 10.2 ± 2.6 for 2-11C-2 and 4-11C-2, respectively. The reproducibility was high for test/retest measures 2 hours apart for 2-11C-2 (data not shown). Direct comparison of the two isopotomers in the brain in the same animal studied 2 hours apart showed slight differences in pharmacokinetics with the uptake of 2-11C-2 being slightly higher than 4-11C-2 up to 22.5 min which could be accounted for by differences in plasma input. However, after 22.5 min, 2-11C-2 continued to clear while the 4-11C-2 plateaued (Fig. 5 for the global region). This difference was even greater when the time-activity curves were corrected by blood input, suggesting the entry of a labeled metabolite into the brain (data not shown).
Fig. 4.
The PET image in baboon summed from time of injection through 90 min for (A) 2-11C-2 (injected dose = 2.96 mCi), (B) 11C-3 (injected dose = 3.42 mCi), and (C) 11C-4 (injected dose = 3.27 mCi). Ketamine hydrochloride (10 mg/kg) was used as an anesthesia by an intramuscular injection. We note that the distribution of 4-11C-2 is not shown as the images do not differ between 4-11C-2 and 2-11C-2.
Fig. 5.
Time-activity curves for the global uptake of [2-methoxy-11C] GTS-21 (2-11C-2) and [4-methoxy-11C] GTS-21 (4-11C-2).
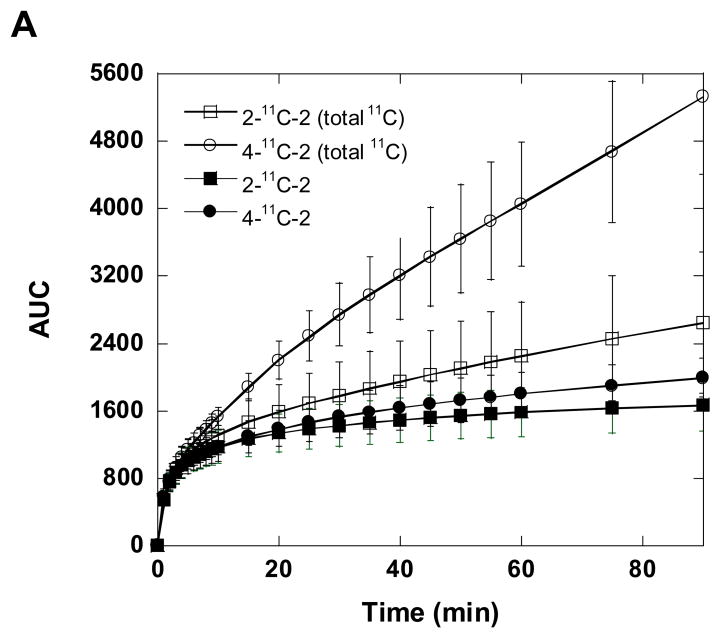
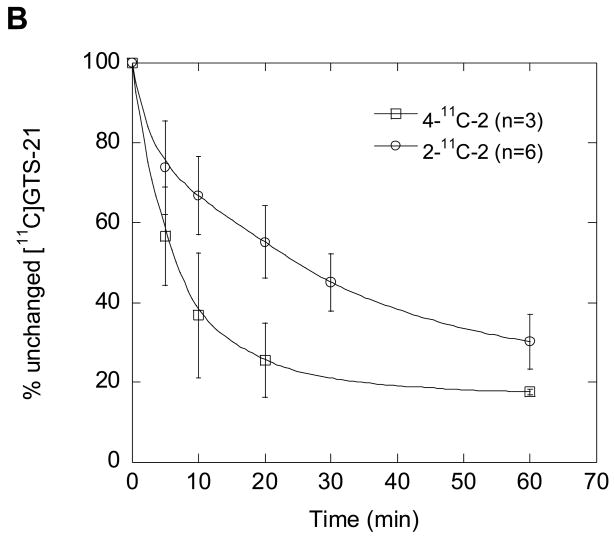
We also measured the total C-11 concentration in the plasma over the time course of each study and performed HPLC analysis of selected samples to determine the fraction present as parent labeled compound. The recoveries of C-11 from HPLC effluent based on injected plasma sample were ≥ 75% (decay corrected). The total radioactivity concentration in plasma was consistently higher for the 4-11C-2 than for 2-11C-2 from 15 min to the end of the study as assessed by the area under the time-activity curve (Fig. 6A; P=0.004 at 90 min). However, the % of unchanged parent compound by HPLC is lower for 4-11C-2 than for 2-11C-2, consistent with more rapid demethylation of the 4-methoxy group (Fig. 6B) [31]. After the total C-11 for each isotopomer was corrected for the fraction that is present as the parent isotopomer, the areas under the plasma time-activity curves for 2-11C-2 and 4-11C-2 at 90 min were similar (Fig. 6A; P= 0.2).
Fig. 6.
(A) The time-AUC (the area under the curve) plots of averaged total carbon-11 in baboon plasma (closed symbols) and [11C]GTS-21 (open symbols) after injection of. 2-11C-2 and 4-11C-2. (B) The percent (%) of unchanged [11C]GTS-21 for 2-11C-2 and 4-11C-2 in baboon plasma. These data were the averaged values obtained after 4-11C-2 (n= 3) and 2-11C-2 (n= 6) administration in three baboons.
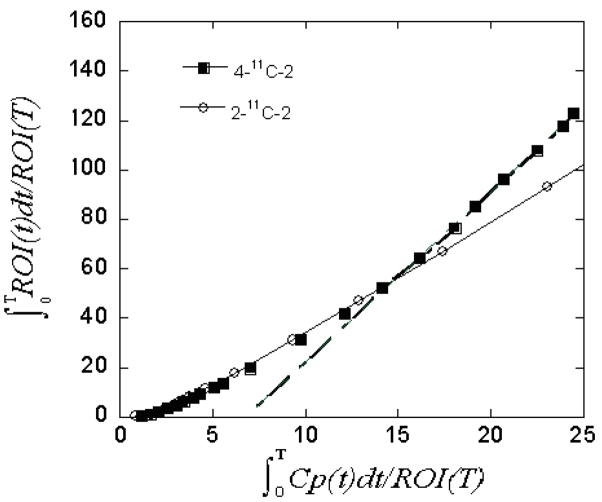
Time-activity curves in the brain and plasma integrals were used to compare the Logan plots for the two isotopomers. The Logan plots paralleled each other until 27 min after which time the slope was clearly steeper for 4-11C-2 (Fig. 7) indicating a contribution by a labeled metabolite of GTS-21, presumably 11C-4 produced by the demethylation of 4-11C-2.
Fig. 7.
Logan plots for two isotopomers of [11C]GTS-21.
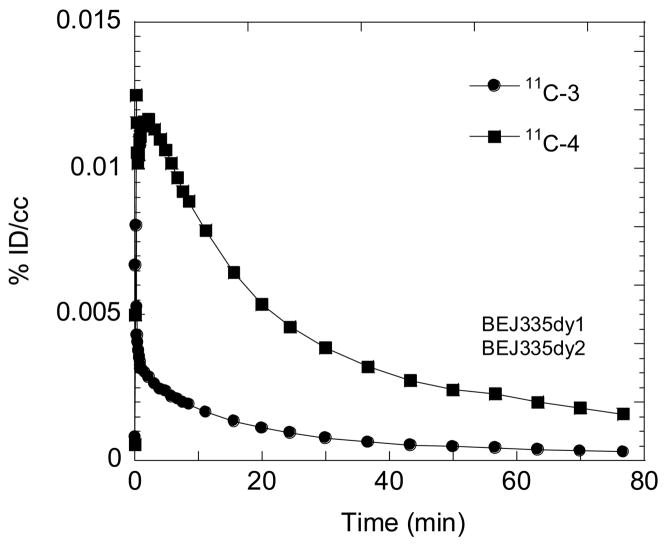
The observation of differences in the time activity curves for the brain and the plasma for 2-11C-2 and 4-11C -2 stimulated the idea of radiolabeling of their respective metabolites 11C -3 and 11C-4 to compare their BBB penetration. In a comparison in the same baboon, the global uptake of 11C-4 was moderate (0.012%ID/cc at 2.5 min) and 4 times greater than 11C-3 (Fig. 8) though 2 times lower than [11C]GTS-21 itself (Fig. 5). Thus, the brain uptake of carbon-11 after the injection of 11C-4 is higher than 11C-3 even though both have similar log D and 11C-4 has a lower free fraction in plasma than 11C -3. 11C -3 and 4 have similar areas under the plasma time-activity curves (data not shown). These results and the known pharmacological activity of 2-OH-GTS-21 (4) at human α7 nAChR in in vitro studies support strongly its consideration as a therapeutically active metabolite of GTS-21. We note that the higher BBB penetration of 11C-4 that we observed in the baboon contrasts with a prior study in the rat which reported that 3 has a high BBB penetration than 4 [31]. Species differences, timing, multiple dosing in the rat and different routes of administration may account for these differences.
Fig. 8.
Time-activity curves for the global uptake of two labeled metabolite of GTS-21, 11c-3 and 11C-4, in the same baboon 2 hours apart.
Since GTS-21 is a drug candidate, it would be given in a therapeutic dose which might be expected to change its pharmacokinetics. 2-11C-2 was chosen rather than 4-11C-2 for the study with the co-administration of GTS-21 because our studies showed that 2-11C-2 better reflects the pharmacokinetics of the labeled drug, without the confounding effect from the labeled metabolite. In comparison with baseline and co-administration of a therapeutic dose of GTS-21 [21] with 2-11C-2, we found that it reduced brain uptake in the initial 5–10 minutes for the cerebellum and the thalamus. The AUC for the plasma after co-administration was 17–23% higher than baseline possibly due to drug occupancy of peripheral binding sites [12]. We applied the Logan graphical analysis to estimate the blood to tissue transfer term (K1) for thalamus, putamen and cerebellum which were decreased by 20–40%, indicating a global decrease in blood flow induced by the intravenous administration of GTS-21 (Table 2). We also estimated the distribution volume for these brain regions and found a uniform reduction of 17–22% (Table 2). Even though the distribution of nAChR in the brain is heterogeneous [40,41], the reduction in distribution volume across brain regions indicates a non-specific effect consistent with the low binding affinity of GTS-21.
Table 2.
Effect of a therapeutic dose of GTS-21 (0.031 mg/kg)* in the baboon brain
| Region of interest | Baseline | GTS-21 | Percent difference (%) | |||
|---|---|---|---|---|---|---|
| K1 | DV | K1 | DV | K1 | DV | |
| Cerebellum | 1.12 | 3.86 | 0.88 | 3.19 | −21.4 | −17.4 |
| Caudate | 1.03 | 4.42 | 0.74 | 3.57 | −28.2 | −19.2 |
| Putamen | 1.18 | 4.70 | 0.85 | 3.9 | −28.0 | −17.0 |
| Thalamus | 1.21 | 4.83 | 0.72 | 3.75 | −40.5 | −22.4 |
| Visual cortex | 1.33 | 4.25 | 0.98 | 3.43 | −26.3 | −19.3 |
2-11C-GTS-21 was co-injected with GTS-21
Even though there was apparently no specificity due to low signal to noise, the drug concentration in the brain after a therapeutic dose could be derived from % ID/dose of radiotracer. On the basis of the study in which we co-administered 0.031 mg/kg of GTS-21 along with 2-11C-2, we estimated that the global brain concentration of GTS-21 after i.v. administration is 0.5 μM at peak and 0.1 μM at 20 minutes post injection which Machu et al. have considered to be a pharmacologically effective drug concentration [16]. Based on these concentrations and the Ki’s [15] and Bmax’s of the α4β2 [40] and α7 nAChR [41], we estimated that the receptor occupancies of α4β2 and α7 nAChR by GTS-21 would be 85 and 70% at peak uptake and 60 and 36% at 20 min, respectively.
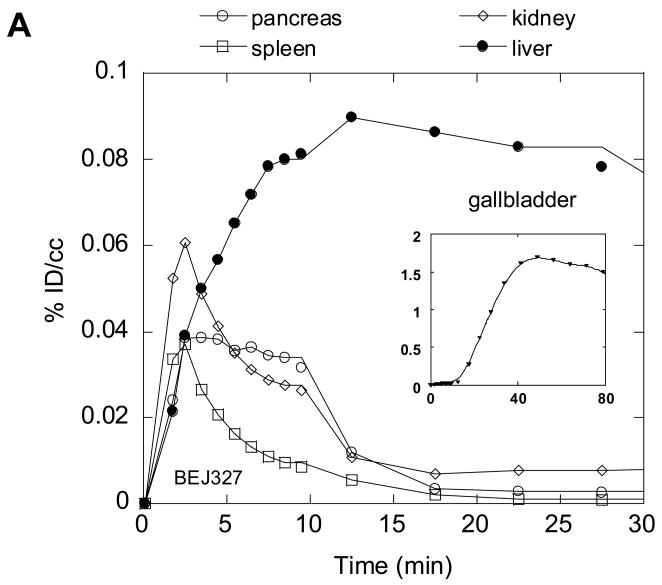
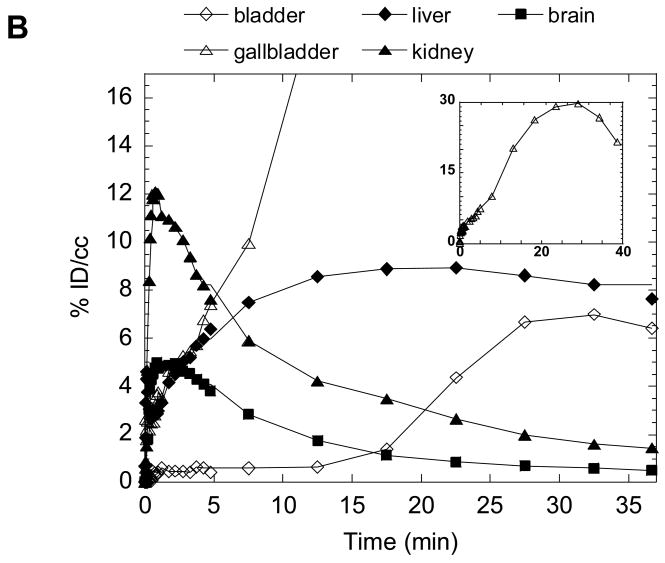
Because drug disposition in peripheral organs can be a source of side effects and toxicity, we also performed dynamic PET studies to determine the biodistribution of 2-11C-2 in peripheral organs. The distribution of C-11 in peripheral organs after the injection of 2-11C-2 into baboon showed that the main excretion route was through the biliary tract (Fig. 9(A)). The liver uptake increased to 0.08 %dose/cc at 22 min and decreased gradually while the gallbladder steadily accumulated C-11, reaching a dose of 1.7 %dose/cc at 49 min, consistent with the previous report that GTS-21 is cleared mainly by liver [42]. Whole body dynamic PET studies in the mouse gave similar results to baboon with rapid brain uptake, a gradual increase in liver and excretion through the gallbladder (Fig. 9(B)). We note that in mouse PET imaging the whole body is in the field of view so that drug pharmacokinetics in the brain and peripheral organs are obtained simultaneously.
Fig. 9.
Time-activity curves for the baboon torso (A) and the whole body of mouse (B) after administration of 2-11C-2.
HPLC analysis of mouse brain homogenate at 17.5 min post injection showed 0.3 %Dose/cc which is consistent with micoPET study (0.25 %dose/cc, data not shown). The major fraction of the total C-11 in the mouse brain was 2-11C-2 (86%). Its labeled metabolite, 11C-3 in the brain was less than 3%. However, 2-11C-2 and its demethylated metabolite, 11C-3 were 23% and 42% of the total C-11 in the plasma, respectively, suggesting that 11C-3 in the brain may be due to the presence of blood in the brain. Ex vivo mouse studies of 2-11C-2 and 4-11C-2 were consistent with PET data, showing high uptake in the gallbladder, liver, small intestine (data not shown).
4. Conclusion
Labeled drugs are of intrinsic interest in that they allow the direct assessment of drug pharmacokinetics. Of particular relevance for CNS drugs are BBB penetration, drug concentration over time, and the identification of target organs as well as the potential brain penetration of pharmacologically active metabolites. This PET study illustrates the value of comparing different label positions and labeled metabolites to gain insight on the behavior of a CNS drug and its metabolites in the brain. GTS-21 is of interest because it is already being evaluated in humans for its efficacy in improving cognition in schizophrenia [14]. Our main findings from this PET study in baboons are that (1) GTS-21 has a very high initial uptake in both cortical and subcortical brain regions followed by rapid clearance; (2) 2-OH-GTS-21, one of the pharmacologically active metabolites of GTS-21, penetrates the BBB; (3) the binding in brain of C-11 labeled GTS-21 (2-11C-2) was only transiently affected by the co-administration of a therapeutic dose of GTS-21 with little specificity, consistent with the relatively low affinity of GTS-21 for nAChR and indicating that its distribution and kinetics is dominated by non-specific binding; (4) GTS-21 and its labeled metabolites are excreted via the hepatobiliary pathway. This study also showed that GTS-21 labeled in the 2-methoxy position is more suitable for the brain pharmacokinetic study of GTS-21 itself than GTS-21 labeled in the 4-methoxy group as this latter isotopomer reflects the distribution for both the parent drug and the labeled metabolite. It is clear that carbon-11 labeled GTS, while providing valuable information on the pharmacokinetics of GTS-21 and its metabolites, is not suitable for imaging the nicotinic receptor. This is consistent with its low affinity and specificity. However, based on the results of this PET study, we suggest that this new information on the rapid clearance of GTS-21 from the brain may need to be considered in future applications of this drug in the treatment of neurocognitive disorders. Furthermore, the moderate BBB penetration of the GTS-21 metabolite, 2-OH-GTS-21, suggests that it may contribute to the therapeutic effects of GTS-21.
Acknowledgments
This work was carried out at Brookhaven National Laboratory under contract DE-AC02-98CH10886 with the U. S. Department of Energy and supported by its Office of Biological and Environmental Research and also by the National Institute on Drug Abuse (K05 DA020001). The authors are grateful to Zachary E. Katsamanis, Michael Schueller, Stephen Dewey, Donald Warner, Aarti M. Kriplani, Wynne Schiffer, Martine Mirrione for their advice and discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–91. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Fagerstrom K, Balfour DJ. Neuropharmacology and potential efficacy of new treatments for tobacco dependence. Expert Opin Investig Drugs. 2006;15:107–16. doi: 10.1517/13543784.15.2.107. [DOI] [PubMed] [Google Scholar]
- 3.Court JA, Martin-Ruiz C, Graham A, Perry E. Nicotinic receptors in human brain: topography and pathology. J Chem Neuroanat. 2000;20:281–98. doi: 10.1016/s0891-0618(00)00110-1. [DOI] [PubMed] [Google Scholar]
- 4.Ding YS, Fowler JS. New-generation radiotracers for nAChR and NET. Nucl Med Biol. 2005;32:707–18. doi: 10.1016/j.nucmedbio.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 5.Mazurov A, Hauser T, Miller CH. Selective alpha 7 nicotinic acetylcholine receptor ligands. Curr Med Chem. 2006;13:1567–84. doi: 10.2174/092986706777442011. [DOI] [PubMed] [Google Scholar]
- 6.Berg DK, Conroy WG. Nicotinic alpha 7 receptors: Synaptic options and downstream signaling in neurons. J Neurobiol. 2002;53:512–23. doi: 10.1002/neu.10116. [DOI] [PubMed] [Google Scholar]
- 7.Cilia J, Cluderay JE, Robbins MJ, Reavill C, Southam E, et al. Reversal of isolation-rearing-induced PPI deficits by an alpha 7 nicotinic receptor agonist. Psychopharmacology (Berl) 2005;182:214–9. doi: 10.1007/s00213-005-0069-5. [DOI] [PubMed] [Google Scholar]
- 8.Biton B, Bergis OE, Galli F, Nedelec A, Lochead AW, et al. SSR180711, a Novel Selective [alpha]7 Nicotinic Receptor Partial Agonist: (1) Binding and Functional Profile. Neuropsychopharmacology. 2006;32:1–16. doi: 10.1038/sj.npp.1301189. [DOI] [PubMed] [Google Scholar]
- 9.de Fiebre NC, de Fiebre CM. Alpha 7 nicotinic acetylcholine receptor-mediated protection against ethanol-induced neurotoxicity. Alcohol. 2003;31:149–53. doi: 10.1016/j.alcohol.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Wang HY, Lee DH, D’Andrea MR, Peterson PA, Shank RP, Reitz AB. beta-Amyloid(1–42) binds to alpha7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer’s disease pathology. J Biol Chem. 2000;275:5626–32. doi: 10.1074/jbc.275.8.5626. [DOI] [PubMed] [Google Scholar]
- 11.Young JW, Crawford N, Kelly JS, Kerr LE, Marston HM, et al. Impaired attention is central to the cognitive deficits observed in alpha 7 deficient mice. Eur Neuropsychopharmacol. 2007;17:145–55. doi: 10.1016/j.euroneuro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Kem W, Soti F, Wildeboer K, LeFrancois S, MacDougall K, et al. The nemertine toxin anabaseine and its derivative DMXBA (GTS21): Chemical and pharmacological properties. Marine Drugs. 2006;4:255–73. [Google Scholar]
- 13.Kitagawa H, Takenouchi T, Azuma R, Wesnes KA, Kramer WG, et al. Safety, pharmacokinetics, and effects on cognitive function of multiple doses of GTS-21 in healthy, male volunteers. Neuropsychopharmacology. 2003;28:542–51. doi: 10.1038/sj.npp.1300028. [DOI] [PubMed] [Google Scholar]
- 14.Olincy A, Harris JG, Johnson LL, Pender V, Kongs S, et al. Proof-of-concept trial of an alpha 7 nicotinic agonist in schizophrenia. Arch Gen Psychiatry. 2006;63:630–8. doi: 10.1001/archpsyc.63.6.630. [DOI] [PubMed] [Google Scholar]
- 15.de Fiebre CM, Meyer EM, Henry JC, Muraskin SI, Kem WR, Papke RL. Characterization of a series of anabaseine-derived compounds reveals that the 3-(4)-dimethylaminocinnamylidine derivative is a selective agonist at neuronal nicotinic alpha 7/125I-alpha-bungarotoxin receptor subtypes. Mol Pharmacol. 1995;47:164–71. [PubMed] [Google Scholar]
- 16.Machu TK, Hamilton ME, Frye TF, Shanklin CL, Harris MC, et al. Benzylidene analogs of anabaseine display partial agonist and antagonist properties at the mouse 5-hydroxytryptamine(3A) receptor. J Pharmacol Exp Ther. 2001;299:1112–9. [PubMed] [Google Scholar]
- 17.Zoltewicz JA, Prokaitatrai K, Bloom LB, Kem WR. Long-Range-Transmission of Polar Effects in Cholinergic 3-Arylideneanabaseines - Conformations Calculated by Molecular Modeling. Heterocycles. 1993;35:171–80. [Google Scholar]
- 18.Kem WR. Study of Occurrence of Anabaseine in Paranemertes and Other Nemertines. Toxicon. 1971;9:23. doi: 10.1016/0041-0101(71)90040-7. [DOI] [PubMed] [Google Scholar]
- 19.de Fiebre NC, de Fiebre CM. alpha(7) Nicotinic acetylcholine receptor-mediated protection against ethanol-induced neurotoxicity. Alcohol. 2003;31:149–53. doi: 10.1016/j.alcohol.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Arendash GW, Sengstock GJ, Sanberg PR, Kem WR. Improved Learning and Memory in Aged Rats with Chronic Administration of the Nicotinic Receptor Agonist Gts-21. Brain Res. 1995;674:252–9. doi: 10.1016/0006-8993(94)01449-r. [DOI] [PubMed] [Google Scholar]
- 21.Briggs CA, Anderson DJ, Brioni JD, Buccafusco JJ, Buckley MJ, et al. Functional characterization of the novel neuronal nicotinic acetylcholine receptor ligand GTS-21 in vitro and in vivo. Pharmacol Biochem Behav. 1997;57:231–41. doi: 10.1016/s0091-3057(96)00354-1. [DOI] [PubMed] [Google Scholar]
- 22.Meyer EM, Tay ET, Zoltewicz JA, Meyers C, King MA, et al. Neuroprotective and memory-related actions of novel alpha-7 nicotinic agents with different mixed agonist/antagonist properties. J Pharmacol Exp Ther. 1998;284:1026–32. [PubMed] [Google Scholar]
- 23.Meyer EM, Defiebre CM, Hunter BE, Simpkins CE, Frauworth N, Defiebre NEC. Effects of Anabaseine-Related Analogs on Rat-Brain Nicotinic Receptor-Binding and on Avoidance Behaviors. Drug Development Research. 1994;31:127–34. [Google Scholar]
- 24.Woodruffpak DS, Li YT, Kem WR. A Nicotinic Agonist (Gts-21), Eyeblink Classical-Conditioning, and Nicotinic Receptor-Binding in Rabbit Brain. Brain Res. 1994;645:309–17. doi: 10.1016/0006-8993(94)91665-9. [DOI] [PubMed] [Google Scholar]
- 25.Martin-Ruiz CM, Court JA, Molnar E, Lee M, Gotti C, et al. Alpha4 but not alpha3 and alpha7 nicotinic acetylcholine receptor subunits are lost from the temporal cortex in Alzheimer’s disease. J Neurochem. 1999;73:1635–40. doi: 10.1046/j.1471-4159.1999.0731635.x. [DOI] [PubMed] [Google Scholar]
- 26.Kem WR. The brain alpha7 nicotinic receptor may be an important therapeutic target for the treatment of Alzheimer’s disease: studies with DMXBA (GTS-21) Behav Brain Res. 2000;113:169–81. doi: 10.1016/s0166-4328(00)00211-4. [DOI] [PubMed] [Google Scholar]
- 27.Stevens KE, Kem WR, Mahnir VM, Freedman R. Selective alpha7-nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacology (Berl) 1998;136:320–7. doi: 10.1007/s002130050573. [DOI] [PubMed] [Google Scholar]
- 28.Tatsumi R, Fujio M, Satoh H, Katayama J, Takanashi S, et al. Discovery of the alpha 7 nicotinic acetylcholine receptor agonists. (R)-3′– (5-Chlorothiophen-2-yl)spiro-1-azabicyclo[2.2.2]octane-3,5 ′-[1′,3′]-oxazolidin-2 ′-one as a novel, potent, selective, and orally bioavailable ligand. J Med Chem. 2005;48:2678–86. doi: 10.1021/jm049188d. [DOI] [PubMed] [Google Scholar]
- 29.Kem WR. The brain [alpha]7 nicotinic receptor may be an important therapeutic target for the treatment of Alzheimer’s disease: studies with DMXBA (GTS-21) Behav Brain Res. 2000;113:169–81. doi: 10.1016/s0166-4328(00)00211-4. [DOI] [PubMed] [Google Scholar]
- 30.Meyer EM, Kuryatov A, Gerzanich V, Lindstrom J, Papke RL. Analysis of 3-(4-hydroxy, 2-Methoxybenzylidene)anabaseine selectivity and activity at human and rat alpha-7 nicotinic receptors. J Pharmacol Exp Ther. 1998;287:918–25. [PubMed] [Google Scholar]
- 31.Kem WR, Mahnir VM, Prokai L, Papke RL, Cao XF, et al. Hydroxy metabolites of the Alzheimer’s drug candidate 3-[(2,4-dimethoxy)benzylidene]-anabaseine dihydrochloride (GTS-21): Their molecular properties, interactions with brain nicotinic receptors, and brain penetration. Mol Pharmacol. 2004;65:56–67. doi: 10.1124/mol.65.1.56. [DOI] [PubMed] [Google Scholar]
- 32.Kem RW, Zoltewicz AJ, Meyer Me, Prokai-tatrai K. Anabaseine derivatives useful in the treatment of degenerative diseases of the nervous system. US Patent 5741802. 1998 [Google Scholar]
- 33.Ding YS, Lin KS, Logan J, Benveniste H, Carter P. Comparative evaluation of positron emission tomography radiotracers for imaging the norepinephrine transporter: (S,S) and (R,R) enantiomers of reboxetine analogs ([C-11]methylreboxetine, 3-Cl-[C-11]methylreboxetine and [F-18]fluororeboxetine), (R)-[C-11]nisoxetine, [C-11]oxaprotiline and [C-11]lortalamine. J Neurochem. 2005;94:337–51. doi: 10.1111/j.1471-4159.2005.03202.x. [DOI] [PubMed] [Google Scholar]
- 34.Delrosario RB, Jung YW, Baidoo KE, Lever SZ, Wieland DM. Synthesis and in-Vivo Evaluation of a Tc-99m/99-Dadt-Benzovesamicol - a Potential Marker for Cholinergic Neurons. Nucl Med Biol. 1994;21:197–203. doi: 10.1016/0969-8051(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 35.Black KJ, Snyder AZ, Koller JM, Gado MH, Perlmutter JS. Template images for nonhuman primate neuroimaging: 1. Baboon Neuroimage. 2001;14:736–43. doi: 10.1006/nimg.2001.0752. [DOI] [PubMed] [Google Scholar]
- 36.Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–7. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- 37.Alexoff DL, Shea C, Fowler JS, King P, Gatley SJ, et al. Plasma input function determination for PET using a commercial laboratory robot. Nucl Med Biol. 1995;22:893–904. doi: 10.1016/0969-8051(95)00042-v. [DOI] [PubMed] [Google Scholar]
- 38.Alexoff DL, Vaska P, Marsteller D, Gerasimov T, Li J, et al. Reproducibility of C-11-raclopride binding in the rat brain measured with the MicroPET R4: Effects of scatter correction and tracer specific activity. J Nucl Med. 2003;44:815–22. [PubMed] [Google Scholar]
- 39.Dischino DD, Welch MJ, Kilbourn MR, Raichle ME. Relationship between Lipophilicity and Brain Extraction of C-11-Labeled Radiopharmaceuticals. J Nucl Med. 1983;24:1030–8. [PubMed] [Google Scholar]
- 40.Kulak JM, Musachio JL, McIntosh JM, Quik M. Declines in different beta 2*nicotinic receptor populations in monkey striatum after nigrostriatal damage. J Pharmacol Exp Ther. 2002;303:633–9. doi: 10.1124/jpet.102.039347. [DOI] [PubMed] [Google Scholar]
- 41.Kulak JM, Schneider JS. Differences in [alpha]7 nicotinic acetylcholine receptor binding in motor symptomatic and asymptomatic MPTP-treated monkeys. Brain Res. 2004;999:193–202. doi: 10.1016/j.brainres.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 42.Azuma R, Hirota T, Manabe H, Komuro M, Kiwada H. First-pass of GTS-21 on canine gut wall and liver determined by portal-systemic concentration difference. Eur J Pharm Sci. 2001;14:159–65. doi: 10.1016/s0928-0987(01)00166-x. [DOI] [PubMed] [Google Scholar]