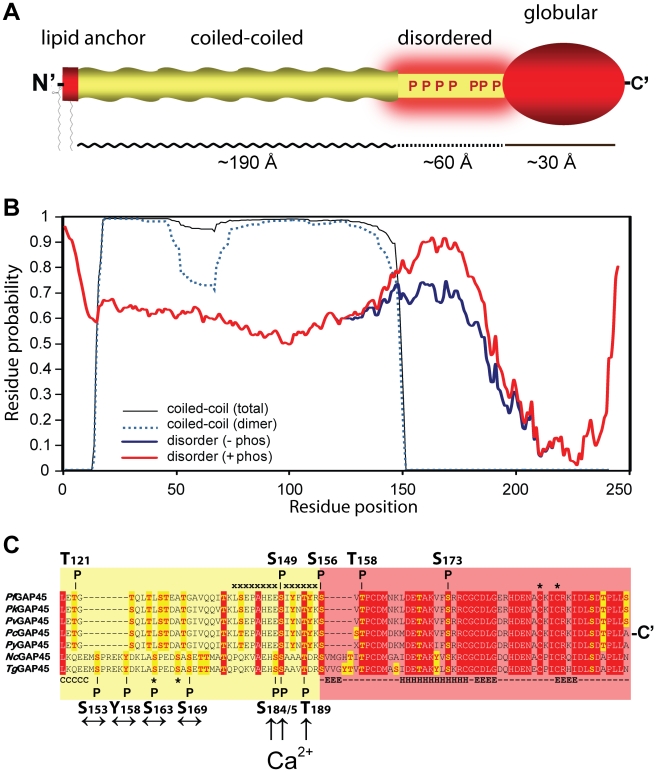
Figure 5. Predicted structure and phosphorylation of Toxoplasma GAP45.
A. Domain model of Toxoplasma GAP45 structure. The protein encodes a short lipid anchored N-terminal domain (aa1-15), an extended alpha-helical coiled-coil domain (aa16–151), a region predicted to be intrinsically disordered (aa152–192) and a globular C-terminal domain (aa193–245). The upper limit of the dimension of the coiled-coil, intrinsically disordered and globular domains derived from structural modeling studies are shown. B. Prediction of the GAP45 coiled-coil and disordered domains. Plots of the overall probability of GAP45 amino acid residues to form a coiled coil (black line) or form a dimeric coiled-coil (broken blue line) are based on the Multicoils program [54]. Plots of the disorder tendency of the unphosphorylated GAP45 amino acid sequence (blue line, -phos) or GAP45 sequence with glutamate replacement of all S/T/Y residues in region aa152-192 are based on the IUPred algorithm [27]. C. ClustalW v.2.1 alignment of GAP45 amino acid sequences from (top to bottom) P. falciparum (PFL1090 w), P. knowlesi (PKH_143920), P. vivax (PVX_123765), P. chabaudi (PCAS_143960), P. yoelii (PY03448), Neospora caninum (NCLIV_048570) and T. gondii (TGME49_023940). Highly conserved residues are highlighted in red and potential S/T/Y phosphorylation sites are highlighted in yellow. The positions of PfCDPK1 in vitro phosphorylation sites of recombinant PfGAP45 identified by Winter et al. (2009) [31](top) and in vivo phosphorylation sites localized on Toxoplasma GAP45 in this study (bottom) are shown. The sequence of a merozoite-derived PfGAP45 phosphopeptide identified by Green et al [22] and the S163 and S167 phosphorylation sites of TgGAP45 are marked (*)[30], and residues that are part of the coiled-coil (C) alpha helices (H) or beta-sheets (E) are annotated. Arrows indicate Ca2+-insensitive (↔) or Ca2+-sensitive phosphorylation sites (↑) on TgGAP45 identified in this study.