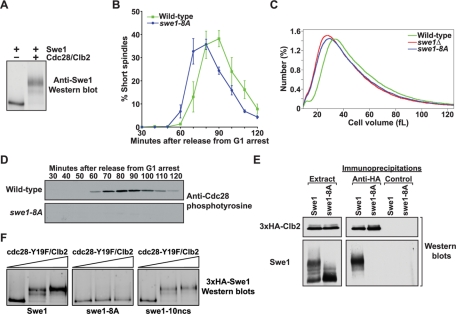
FIGURE 1:
Phosphorylation of Cdc28 consensus sites is necessary for Swe1 activity in vivo. (A) Purified Cdc28/Clb2 quantitatively phosphorylates purified Swe1 in vitro. Phosphorylation of Swe1 causes an electrophoretic mobility shift that is detected by Western blot analysis. (B) Wild-type and swe1-8A cells were released from a G1 arrest, and the percentage of cells with short mitotic spindles was determined at the indicated times and plotted as a function of time. Error bars represent SEM for three independent experiments. (C) Cell size distributions of log-phase cultures of wild-type, swe1Δ, and swe1-8A strains. Each trace is the average of 15 independent cultures. (D) Cells of the indicated genotypes were released from a G1 arrest, and samples were taken at the indicated times. Cdc28 phosphotyrosine was monitored by Western blotting. (E) Wild-type and swe1-8A cells carrying GAL1-3×HA-CLB2 were grown to midlog phase, and expression of 3×HA-Clb2 was induced with 2% galactose for 3 h at 30°C. Extracts were made, and 3×HA-Clb2 was immunoprecipitated with an anti-HA antibody. As a control, identical precipitations were carried out using an anti-GST antibody. Coprecipitation of Swe1 was assayed by Western blotting. The panels labeled “Extract” show Western blots of 3×HA-Clb2 and Swe1 in the crude extracts used for the immunoprecipitations. (F) Purified 3×HA-Swe1, 3×HA-swe1-8A, or 3×HA-swe1-10ncs were incubated with increasing amounts of purified cdc28-Y19F/Clb2-3×HA in the presence of ATP. Phosphorylation of Swe1 was detected as an electrophoretic mobility shift on a Western blot.