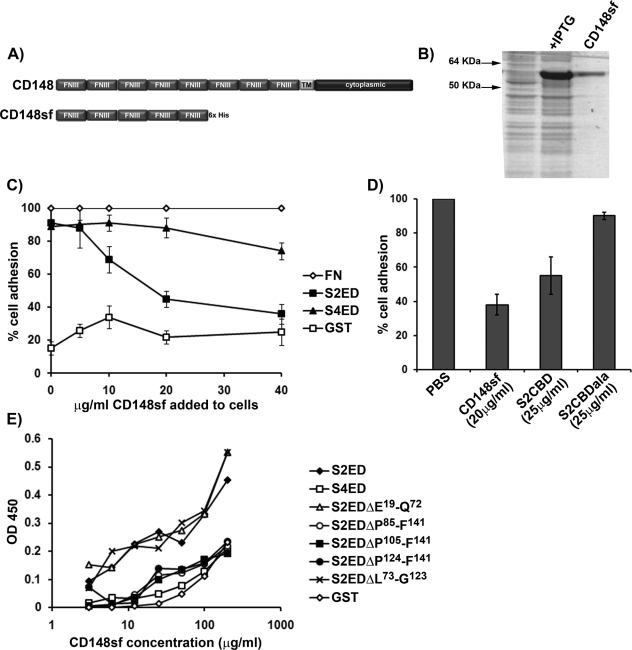
FIGURE 3:
The extracellular domain of CD148 interacts with S2ED. (A) Schematic diagram of CD148 showing the extracellular domain comprising eight FNIII repeats, a single transmembrane domain, and a cytoplasmic domain. The first five N-terminal FNIII repeats were cloned and expressed in E. coli, and the resultant protein is referred to as CD148sf. (B) Purified CD148sf is shown by SDS–PAGE and Coomassie brilliant blue staining. (C) Incubation of Swiss 3T3 fibroblasts in the presence of CD148sf results in compromised cell adhesion to S2ED. Cells were seeded in serum-free media on wells coated with FN, S2ED, S4ED, or GST in the presence of the indicated concentrations of CD148sf. Adhesion is calculated relative to adhesion to FN in the absence of CD148sf. (D) Adhesion to S2ED by primary rat lung fibroblasts also depends on CD148. Cells were seeded on wells coated with S2ED in serum-free media in the presence of CD148sf, the adhesion regulatory peptide S2CBD, and the negative control S2CBDala at the concentrations indicated. Adhesion is calculated relative to adhesion to S2ED in the absence of competitors. (E) Direct interactions between CD148sf and S2ED are mediated through the adhesion regulatory domain of S2ED. Solid-phase binding assays were performed with wells coated with 200 ng of the substrates indicated and incubated in the presence of increasing concentrations of CD148sf as indicated. The amount of bound CD148sf was quantified using antibodies specific for CD148. CD148sf interacts only with S2ED, S2EDΔE19-Q72, and S2EDΔL73-G123, all of which contain the syndecan-2 adhesion regulatory motif.