FIGURE 4:
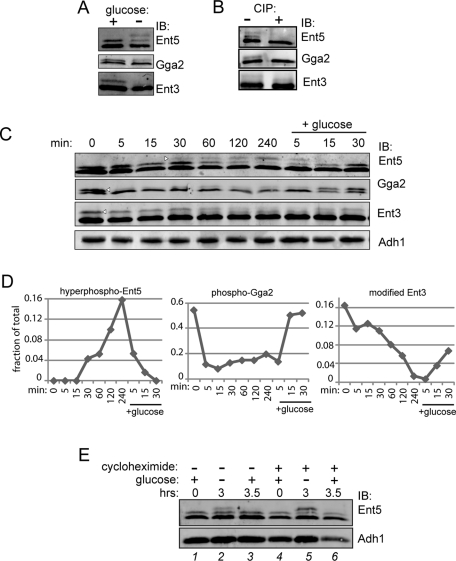
Posttranslational modifications of adaptors are regulated by glucose. (A) Glucose depletion causes hyperphosphorylation of Ent5, dephosphorylation of Gga2, and a change in Ent3 posttranslational modification. Wild-type cells (BY4742) were grown to mid-log phase, then washed with and resuspended in media without glucose for 2 h. Cell lysates were then probed with antibodies to adaptors. (B) Phosphorylation of adaptors. Adaptors were immunoprecipitated from wild-type cells grown to stationary phase (Ent5) or mid-log phase (Gga2 and Ent3). Immunoprecipitated samples were then treated +/− calf intestinal alkaline phosphatase (CIP) and subjected to immunoblot analysis. (C) Time course showing changes in adaptor modification in response to glucose. Wild-type cells were grown to mid-log phase and then washed with and incubated in media without glucose for 4 h. Following starvation, glucose was added to the culture media for 30 min. Samples were taken at indicated time points, lysed, and subjected to immunoblot analysis using antibodies to Ent5, Gga2, Ent3, or Adh1 as a loading control. The fraction of the highest-molecular-weight band (arrowheads) to total was quantitated and is presented graphically in (D). (E) Ent5 phosphorylation does not induce protein turnover during glucose starvation. Cells were grown in the absence (lanes 1–3) or presence (lanes 4–6) of 35 μg/ml cycloheximide to mid-log phase in glucose for 2 h (lanes 1 and 4) and then washed and starved for glucose for an additional 3 h (lanes 2 and 5). Glucose (2%) was then added back to cells for 30 min (lanes 3 and 6).