Abstract
Background and Aims
Low birth weight affects 1 in every 7 babies born globally and can predict a lifetime of increased risk for adverse health outcomes, including cardiovascular disease, hypertension, obesity, diabetes, and metabolic syndrome. Maternal low protein diet during pregnancy and lactation is a well-characterized rat model for low birth weight and the subsequent increase in chronic disease risk. However, mice have been relatively understudied in this paradigm and represent a critical resource for investigating the underlying molecular mechanisms that link adverse early life experience and the development of chronic disease.
Methods and Results
The present manuscript describes a mouse model of low birth weight (maternal consumption of low protein diet (8% protein) through pregnancy and lactation) and characterizes metabolic adaptations (food intake, locomotor activity, oxygen consumption, and glucose tolerance test) in male and female offspring. At weaning, mice were maintained either on the control diet or a high fat diet. Notable sex differences were observed, with male mice from the low protein pregnancies showing increased food intake, hyperactivity and increased metabolic rate only when weaned to the high fat diet, while female mice consistently showed increased food intake and were hypometabolic, regardless of postweaning diet.
Conclusion
These data identify offspring sex and postweaning diet as critical variables in the metabolic adaptations to early life protein deficiency, and suggest that females may be more vulnerable to the adverse long term health consequences of low birth weight.
Keywords: in utero programming, obesity, activity, metabolism
Introduction
The “fetal origins” hypothesis developed by Barker and colleagues noted the relationship between inadequate maternal nutrition and the eventual development of metabolic disease (hypertension, insulin resistance, and dyslipidemia) in adulthood (1). This relationship has been seen across diverse examples of human famine, including the Dutch Hunger Winter (2) and the 1950s Chinese Famine (3, 4). To varying degrees, this finding has been replicated in animal models, as aspects of the metabolic syndrome (hypertension, atherosclerosis, dyslipidemia, glucose/insulin imbalance, central adiposity) can be seen in the adult offspring born to dams that consume limited nutrition during pregnancy and/or lactation. Maternal protein restriction (typically restricted to half the normal protein levels) has been extensively studied as a valid rodent model for the development of low birth weight (growth-retarded) offspring (5–9). The vast majority of the protein-restriction experiments have been done using rats, and relatively few studies to date (10–12) have examined the effects of maternal protein restriction in a mouse model. Further, significant sex differences have been noted in the offspring’s response to prenatal undernutrition (13–17). For example, male mice from undernourished dams were adversely affected with respect to weight gain, adiposity and glucose tolerance (13), effects which were all absent in females. From these and other studies, it is clear that species, sex and the timing of the restriction (pregnancy and/or lactation) exhibit critical interactions that impinge on the outcome of study. Given the power of mouse genetics, it is critically important to fully characterize the effect of maternal protein restriction in a mouse model; therefore, current experiments were designed to examine the effect of maternal protein restriction on metabolic endpoints in male and female mouse offspring.
Methods
Animals and experimental model
Eight to 12 week old C57BL/6J females (The Jackson Labs, Bar Harbor, ME) were mated with DBA/2J males (The Jackson Labs, Bar Harbor, ME) and fed either a control (18% protein) or isocaloric 8.5% low protein (LP) diet during breeding, pregnancy and lactation (diet details below, and see Supplemental Table). Breeding pairs were housed in standard mouse cages with the addition of nestlets and breeding houses; males were removed from the pair just prior to the birth of the pups. Litters with fewer than 6 or greater than 8 pups were excluded from analysis. At weaning, half the litters were fed the control diet, and half were fed a high-fat diet (60% calories from fat). One animal per litter from 6–8 different dams/group was randomly chosen for use in individual experiments, to control for any litter effect. There was no difference in litter size between LP and control pregnancies. Body weights were recorded weekly, and male and female mice (n=4–6/group) were used in all experiments. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania.
Diet Composition
Total energy content of control diet (Test Diet 5755, Richmond, IN) was 4.09 kcal/g with 18% of total energy calories from protein, 22% from fat, and 60% from carbohydrate. The isocaloric, low protein diet (Test Diet 5769) was 8.5% protein purified diet with total energy content of 4.13 kcal/g with 8.5% of total energy calories from protein, 22% from fat, and 69.5% from carbohydrate. High fat diet (Test Diet 58G9) had a total energy content of 5.21 kcal/g with 18% of total energy calories from protein, 60% from fat, and 22% from carbohydrate.
Metabolic measurements
Animals were tested in the Comprehensive Lab Animal Monitoring System (CLAMS, Columbus Instruments, Columbus, OH), which monitors food and water intake, indirect calorimetry, and x-axis activity. Animals had unrestricted access to powdered chow through a feeder located in the middle of the cage floor, which was directly connected to a precision scale. At least one week prior to testing, animals were housed in the CLAMS overnight to acclimate to powdered food and a novel cage. While in the CLAMS cages, animals had ad libitum access to powdered diet and water. Food intake was normalized to body weight. Animals were placed in the cages one hour prior to lights out and were removed 20 hrs later. Data from the 12 hr dark period and the first 6 hrs of the light period were analyzed and reported. Data in Figure 2D were previously published in (18).
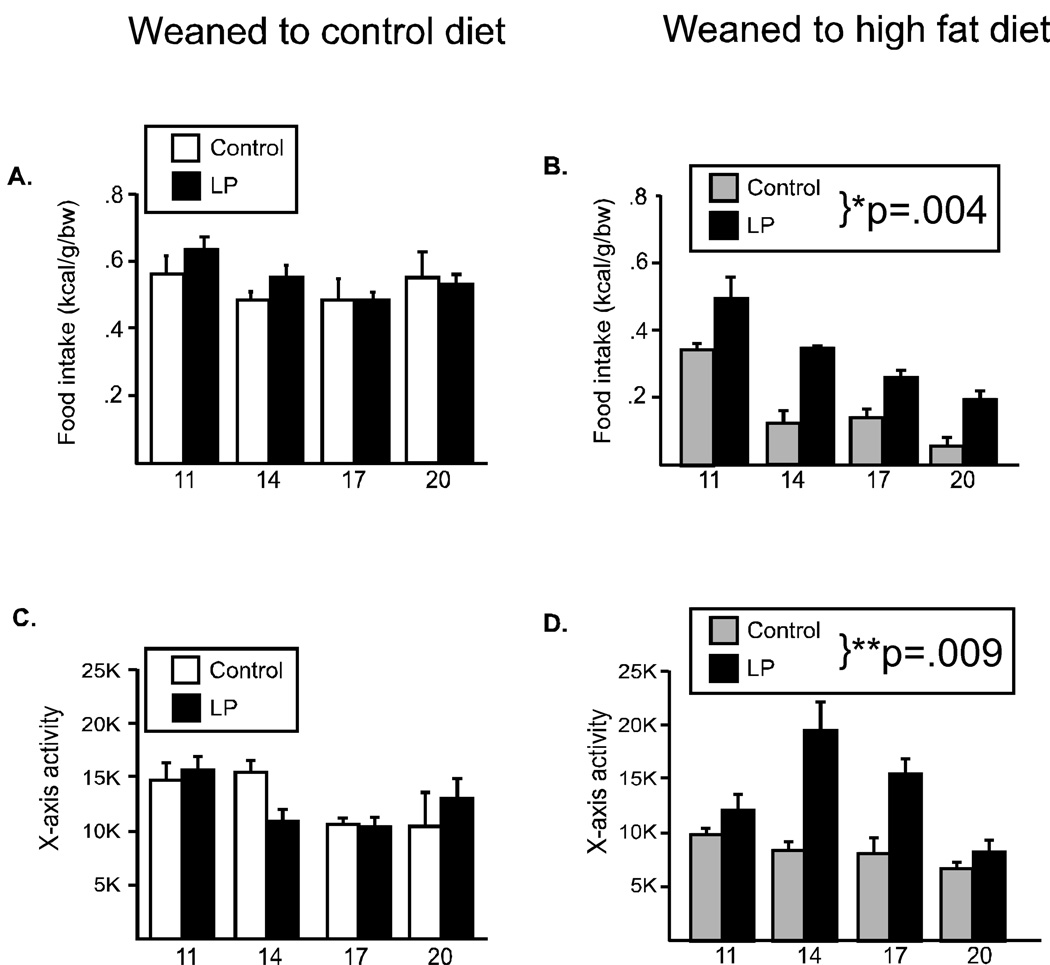
Figure 2. Food intake and locomotor activity in male offspring.
Food intake (A, B) and locomotor activity (C, D) were evaluated every three weeks in male offspring from 11 through 20 weeks of age. When offspring were weaned to the control diet, there were no differences due to maternal diet (A, C). However, when offspring were weaned to the high fat diet (HF), the offspring from dams fed the low protein diet had a significantly increased food intake (B) and were hyperactive (D). (*p=.004, **p=.009)
Glucose tolerance test
An intraperitoneal glucose tolerance (IPGTT) test was performed at 3 months (males) or 6 months (females) of age after an 18-hr overnight fast (n=5/group). A glucometer (Ascensia, Bayer) was used to determine blood glucose levels using blood from a tail nick. After determination of fasting blood glucose levels, each animal received a 2g/kg intraperitoneal injection of 20% glucose. Blood glucose levels were detected after 15, 30, 60, and 120 min. Glucose tolerance was calculated by adding the areas under the glucose-time curve (AUC-GTT) for the intervals 0–15, 15–30, 30–60, and 60–120 min.
Statistical analyses
Data, presented as means ± s.e.m., were analyzed using Prism 4 (GraphPad) and Excel tools for statistical analysis. Student’s t-test was used to analyze differences between groups in Fig 1B–E. Two-way repeated measures ANOVA (maternal diet × time) was used to analyze all other data sets. A p-value of 0.05 or lower was considered significant.
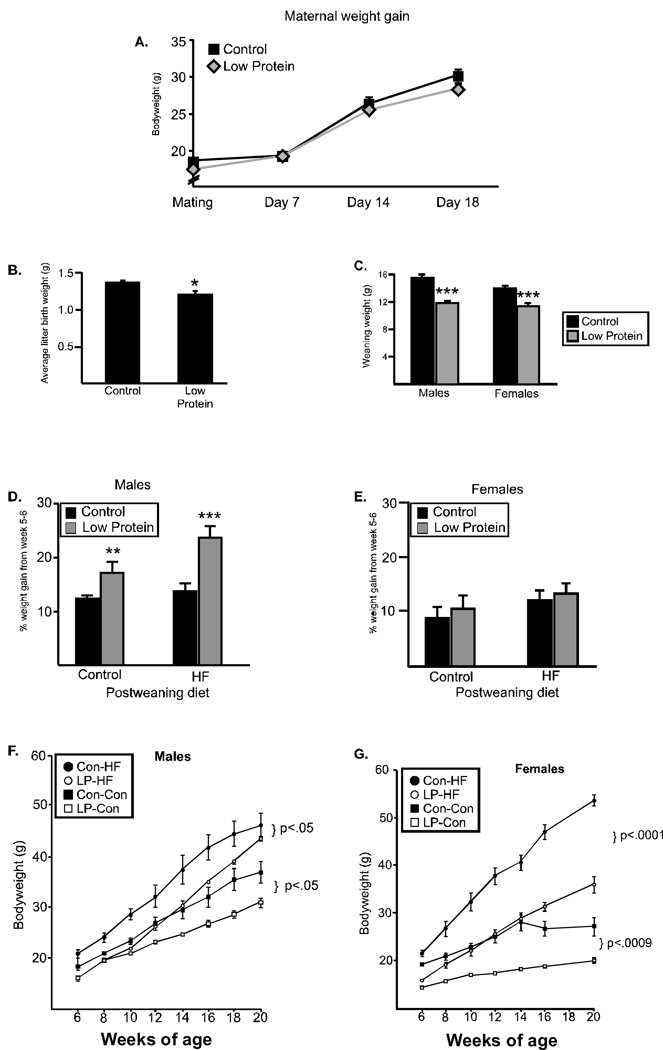
Figure 1. Dam and offspring weight gain.
(A) Maternal weight from the time of mating through 18 days of pregnancy did not differ between dams fed the low protein diet (gray diamond) and dams fed the control diet (black square). (B) Average litter birth weight was significantly reduced when dams were fed the low protein diet. (C) Weaning weight for both males and females was significantly lower in offspring from dams fed the low protein diet (gray) as compared to control offspring (black). (D) Percent weight gain between weeks 5 and 6. Males from low protein pregnancies gained significantly more weight than controls, however, the rate of weight gain for females (E) did not differ between groups (*p<.05, **p<.008, ***p<.0005). Offspring weight gain over time in males (F) and females (G). Offspring from dams fed the low protein diet during pregnancy and lactation (gray) weighed less than controls (black) regardless of postweaning diet (control diet (squares) or high fat diet (circles)).
Results
Weights
Dams were fed either a low protein (LP) diet or an isocaloric control diet through breeding, pregnancy and lactation. There was no difference in maternal weight gain during pregnancy between the groups (Figure 1A). At birth, the average weight of each litter was calculated (n=6–8 litters) and the LP offspring weighed significantly less at birth (1.37(0.02) g versus 1.21 (0.04) g, p<.05, Figure 1B). At weaning, both males and females from the LP-fed dams weighed significantly less than offspring from control pregnancies (Males: 11.9 (0.3) vs. 15.6 (0.3), Females: 11.5 (0.3) vs 14.1 (03), p<.0005, Figure 1C). After weaning, pups were placed on either the control diet or a diet high in fat (HF; 60% fat). The rate of weight gain was measured between weeks 5–6 and revealed that males from the LP pregnancies demonstrated significantly greater “catch up” growth than did males from control pregnancies (Figure 1D), on both the control or high fat diet. Females did not differ in their rate of growth, regardless of postnatal diet (Figure 1E).
Metabolic parameters (weight gain, food intake, locomotor activity and metabolic rate) were examined every 3 weeks in a subset of animals (n=4–5 /group) from 9–20 weeks of age. All animals gained weight over time, with a significant (p<.0001) effect for time for all groups. Regardless of the postweaning diet, male offspring from the LP pregnancies maintained a lower body weight from 6–20 weeks of age (Main effect for group; Control diet: F(1,42)=9.3, p<.05; HF diet: F(1, 35)=6.4, p=.05, Figure 1F). A similar pattern of weight gain was observed in the females. Regardless of the post-weaning diet, female offspring from LP pregnancies weighed significantly less than offspring from control pregnancies (Main effect for group: Control diet: F(1,36)=36.7, p<.0009, HF diet: F(1, 36)=74.4, p<.0001, Figure 1G).
Males-Dark period
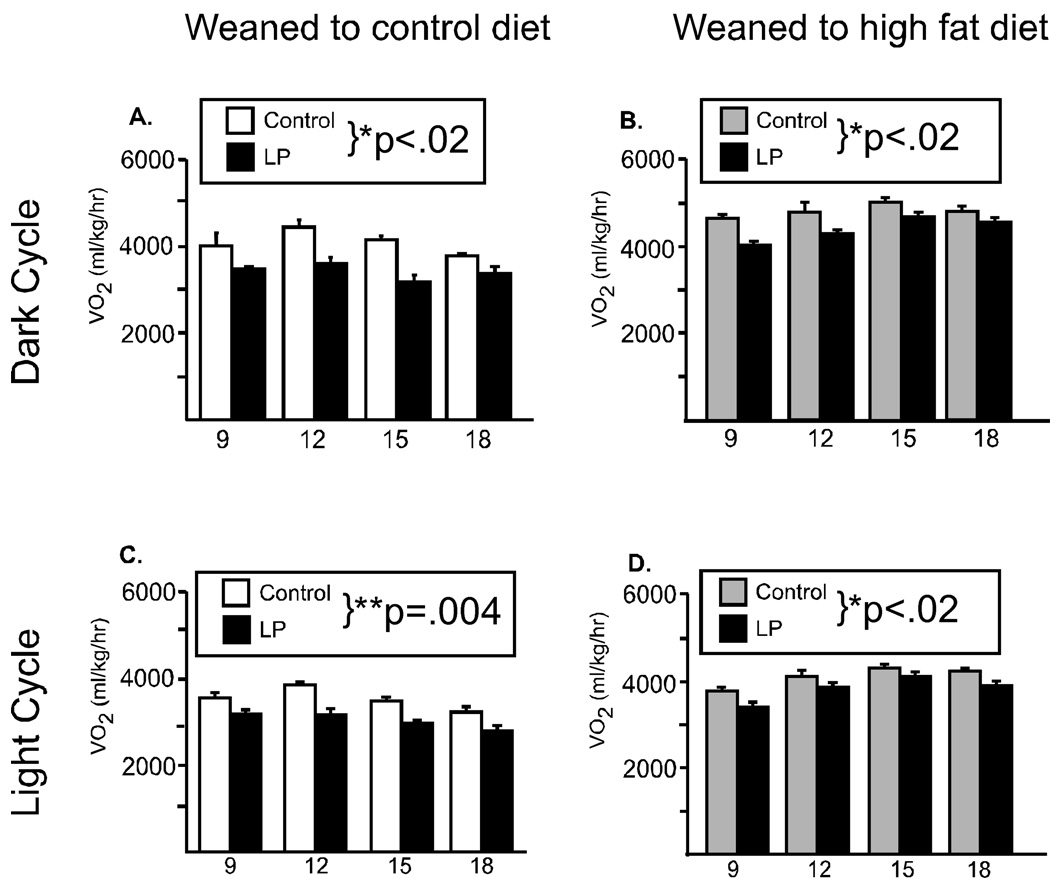
Male mice from LP pregnancies did not differ in food intake or locomotor activity from their control counterparts when weaned postnatally to the control diet (Figure 2A, 2C). However, when animals were presented with a metabolic challenge (consumption of the high calorie, high fat diet) significant differences were observed. Repeated measures ANOVA revealed that male mice from the LP pregnancies ate significantly more food compared to mice from the control pregnancies (main effect for group: F(1,12)= 33.5, p=.004, Figure 2B) and demonstrated a significant hyperactivity (F(1,15)=16.8, p<.009, Figure 2D). Similarly, oxygen consumption (an indicator of basal metabolic rate) did not differ between the groups when animals were weaned to the control diet (Figure 3A). However, when offspring were weaned to the high fat diet, there was a significant increase in metabolic rate, such that animals from the LP pregnancies had a significantly greater oxygen consumption (F(1,18)=7.98, p=.03, Figure 3B).
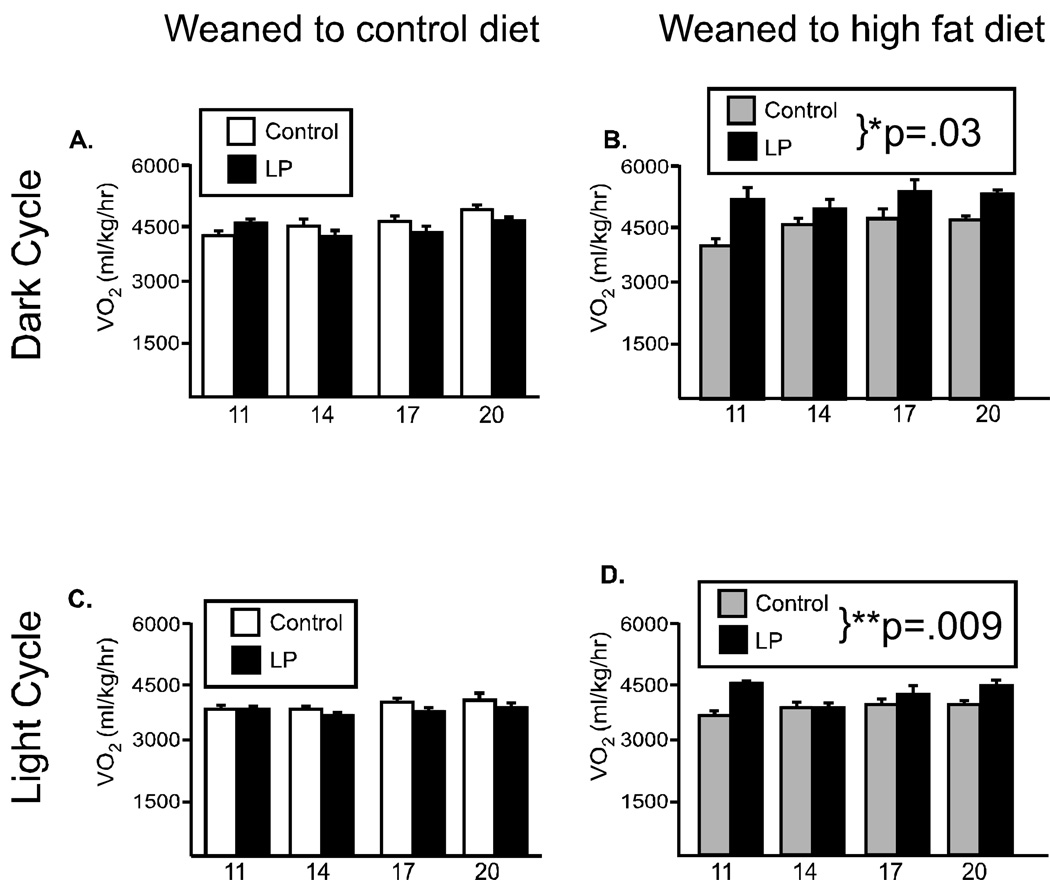
Figure 3. Oxygen consumption in male offspring.
Metabolic rate was evaluated every three weeks in male offspring from 11 through 20 weeks of age, during the lights off period (6pm-6am, A, B) and the first 6 hrs of the lights on period (6am–12pm, C, D). When offspring were weaned to the control diet, there were no differences due to maternal diet (A, C). However, when offspring were weaned to the high fat diet (HF), the offspring from dams fed the low protein diet had significantly increased oxygen consumption (B, D). (*p<.03, **p=.009)
Light period
As expected for a nocturnal animal, food intake and locomotor activity were significantly reduced during the lights on period, but neither food intake nor locomotor activity differed between the groups during the lights on period (data not shown). However, as in the dark period, oxygen consumption was significantly elevated in animals from the low protein-fed dams (F(1,18)=14.7, p=0.009, Figure 3D) when weaned to the HF diet, while there was no group difference in animals fed the control diet (Figure 3C).
Comparison between postnatal diets
It is also informative to examine the two groups’ response to the presence of HF diet. Both LP and control offspring decreased food intake in the presence of high fat diet, although less so for the LP offspring. However, for both locomotor activity and oxygen consumption there were notable differences. Activity level in control offspring decreased in the presence of a high fat diet, however in offspring from the LP pregnancies, activity levels were significantly increased, particularly in the 14–17 week timeperiod. Further, oxygen consumption did not change in the presence of the high fat diet for controls, but LP-derived offspring had an elevated VO2 in the presence of the HF diet.
Females
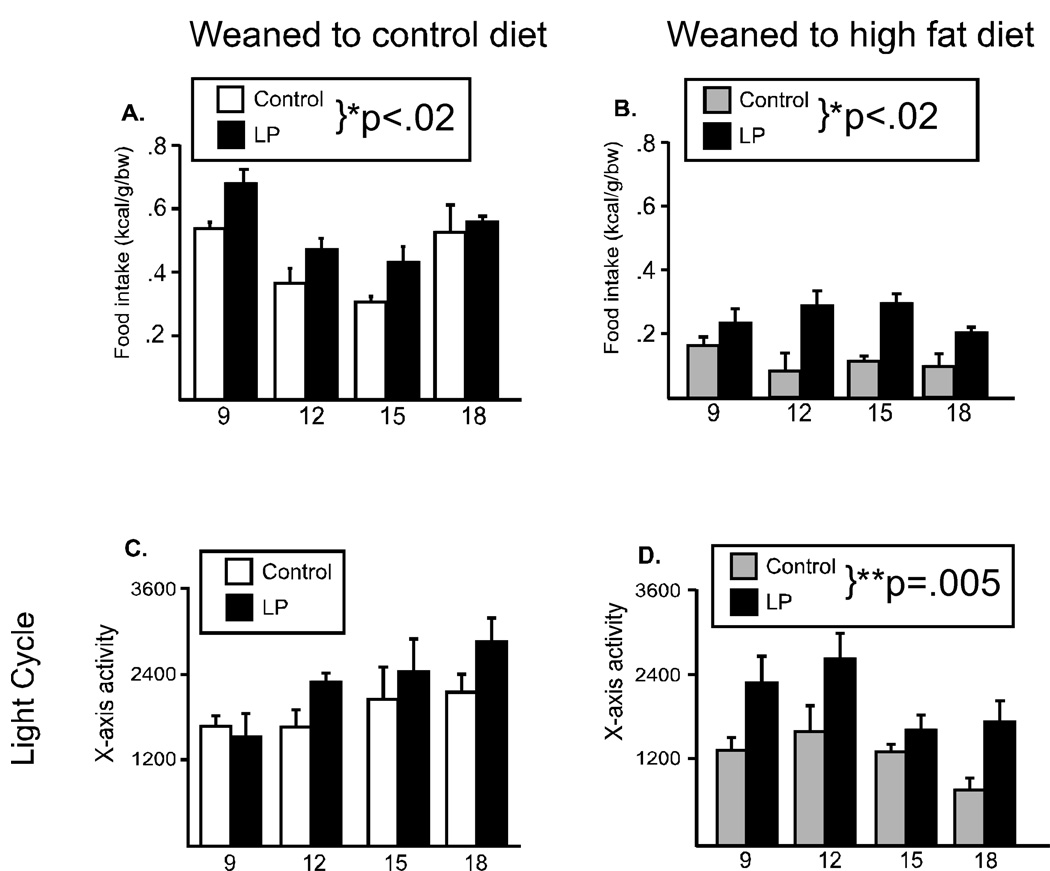
The pattern of metabolic responses observed in females was notably different than that observed in the males. With regard to food intake, female offspring from dams that were fed the LP diet during pregnancy and lactation ate significantly more food, regardless of the postweaning diet (Main effect for group; Control diet: F(1,24)=10.3, p=.0184, Figure 4A; HF diet: F(1, 24)=10.9, p=.0165, Figure 4B). Food intake was significantly less during the lights on period, and did not differ between the groups (data not shown). There were no differences in locomotor activity during the dark, regardless of the pregnancy or postweaning diet (data not shown), nor during the lights on period when animals were fed the control diet (Figure 4C). However, during the lights on period, x-axis activity was significantly increased in LP offspring weaned to the HF diet (F(1, 18)=18.7, p=.005, Figure 4D). Metabolic rate was significantly reduced in offspring from LP-fed dams, regardless of the postweaning diet, in both the dark and light period (Dark: Main effect for group; Control diet: F(1,18)=12.3, p=.0127, Figure 5A; HF diet: F(1, 15)=14.2, p=.013, Figure 5B, Lights on; Control diet: F(1,18)=19.6, p=.004, Figure 5C; HF diet: F(1, 18)=11.1, p=.016, Figure 5D). In contrast to males, the female offspring’s response to the high fat diet did not differ between the groups. Weaning to the high fat diet, decreased food intake, decreased locomotor activity and increased metabolic rate in both control and growth restricted female pups.
Figure 4. Food intake and locomotor activity in female offspring.
Food intake (A, B), locomotor activity (C, D) were evaluated every three weeks in female offspring from 9 through 18 weeks of age. During lights off, female offspring from low protein fed dams showed an increase in food intake, regardless of the postweaning diet (A, control diet; B, high fat diet). During lights on animals from the low protein fed dams who were weaned to the HF diet were significantly more active (D). (*p<.02, **p=.005)
Figure 5. Oxygen consumption in female offspring.
Metabolic rate was evaluated every three weeks in female offspring from 9 through 18 weeks of age, during the lights off period (6pm-6am, A, B) and the first 6 hrs of the lights on period (6am-12pm, C, D). Female offspring from low protein fed dams were had significantly lower metabolic rate, regardless of the postweaning diet or the light cycle (A, C, control diet; B, D, high fat diet). (*p<.02, **p=.004).
Glucose tolerance test (GTT)
Intraperitoneal glucose tolerance tests were performed following a 12 hr fast when male mice were 3 months of age and female mice were 6 months of age (Table 1). Male LP offspring had significantly higher fasting glucose levels (control: 102.3 mg/dl (15.8) vs. LP:162 mg/dl (14.8) p<.05), while females did not (control: 154.4 (6.6) vs. LP: 150.2 (5.5). As expected, exposure to the HF diet significantly increased fasting blood glucose levels (in all groups of animals), but the increase was not significant in the LP offspring due to the increased fasting glucose levels when on the control diet. Area under the curve for the IPGTT did not differ in males or females, regardless of postweaning diet.
Table 1.
Glucose tolerance test in males (3 months) and females (6 months).
| MALES | Maternal Diet | ||
|---|---|---|---|
| Postweaning diet | Control | Low Protein | |
| Fasting glucose | Control | 102.3 (15.8) | 162.0 (14.8)* |
| High fat | 199.7 (26.3)† | 178.5 (17.3) | |
| AUC-IPGTT | Control | 874.2 (88) | 810.7 (32) |
| High fat | 988.4 (88) | 1038.2 (78.5) | |
| FEMALES | Maternal Diet | ||
| Postweaning diet | Control | Low Protein | |
| Fasting glucose | Control | 154.4 (6.6) | 150.2 (5.5) |
| High fat | 176 (5.2)† | 186.4 (13.8)† | |
| AUC-IPGTT | Control | 593.2 (29.8) | 636.3 (23.5) |
| High fat | 725.7 (62.6) | 682.7 (22.9) | |
Glucose tolerance test performed after 18hr fast. Male mice from LP pregnancies demonstrate a significantly elevated fasting glucose level when maintained on the control diet from weaning compared to mice from control pregnancies (* p<.05, mean ± S.E.M.).
Animals weaned to the high fat diet had significantly increased fasting glucose levels as compared to animals on the control diet in the glucose tolerance test, as indicated (†p<.05, mean ± S.E.M.).
Discussion
In the present manuscript, we describe metabolic consequences of early life protein restriction that vary by sex and postweaning diet. Maternal protein restriction in mice led to offspring that were low birth weight and remained lower birth weight through 20 weeks of age. This growth pattern mirrors the pattern seen in a similar mouse model of maternal protein restriction (where the protein restriction was limited to pregnancy only (13)), as well as in rat studies in which the protein restriction encompassed pregnancy and lactation (5–8). Males, but not females, demonstrated catch-up growth on both the control and high fat diet. In male offspring, there was an interaction between maternal diet and postweaning diet, such that after chronic consumption of high fat diet, LP males had increased food intake, were hyperactive and had increased metabolic rate, as compared to control offspring. Hyperactivity and increased metabolic rate may compensate for the increased food intake and partially explain why the low protein offspring did not weigh significantly more than the controls. These data are particularly interesting given that an increased risk for attention deficit hyperactivity disorder (ADHD) has been linked (independently) to both small size at birth (19, 20) and overweight (21).
Importantly, the impact of maternal low protein diet during pregnancy and lactation on metabolic parameters was dependent on sex. In contrast to the males, females from the low protein pregnancies ate significantly more food and were hypometabolic, regardless of the postweaning diet. Female offspring from the low protein pregnancies continued to weigh less than control offspring, which is surprising given the observed increase in food intake coupled with lowered metabolic rate. It remains possible that a longer assessment would reveal an eventual increased weight gain relative to controls in the low protein offspring. Findings using similar protein restriction models show increased body weight in LP females only beyond 6 months of age (10), the time at which the current study was concluded, or even later at 32 weeks of age (13). Further, increased adiposity has been reported in the absence of a differential weight gain (16), and it would be important for future studies with female offspring from low protein pregnancies to examine independent measures of adiposity in addition to body weight. The mechanistic explanation for the observed sex difference is beyond the scope of the current study, but known sex differences in metabolic regulation (22) and the interaction of sex hormones with food intake, activity and metabolic rate are likely to play an important role.
The developmental window during which protein restriction occurs is a critical variable affecting the development of adverse effects in the offspring. Because related studies in our laboratory focus on the effects of early life protein restriction on brain development (18), we include protein restriction during both pregnancy and lactation, as rodent brain development through postnatal days 7–10 is similar to the third trimester of human pregnancy. Further, a number of seminal papers using the rat model that examine growth and metabolic phenotypes have used protein restriction through pregnancy and lactation (23–25). A comparison of the present results to those in a mouse model in which protein restriction was limited to only pregnancy identified similarities as well as differences. Similar to the present findings, eight week old LP male offspring ate more food, were more active during the dark phase, and had increased energy expenditure during lights on (11). However, in our model, these metabolic changes were only observed once animals were challenged with chronic exposure to the high fat diet. Further, offspring from protein-restricted dams in our study weighed less than controls, regardless of diet, while animals in the other study gained significantly more weight than controls when put on a high fat diet starting at 8 weeks of age. It may be the case that extension of the protein restriction to the lactation period is the reason for this difference, as previous studies have reported that when protein restriction was limited to the lactation period only, there was a net beneficial effect, as mice lived longer, weighed less, and were not affected by a high fat diet (26). The fathers were also exposed to the LP diet, however, a contribution from the paternal nutritional history to the observed phenotypes is unlikely given that females became pregnant within 0–4 days of pairing, a timeframe during which novel spermatogenesis would not occur (i.e., > 20 days).
Results from this mouse model appear to have notable differences with observations in the rat. The bodyweight phenotype is similar in mice and rats, with LP offspring born low birthweight (23, 27) and remaining lower body weight (5–8). However, when examining metabolic adaptations, clear differences emerge. Increased food intake was not observed in either males or females in a similar rat model in which protein restriction was continued through pregnancy and lactation (7). An early but transient increase in food intake (present at ~35–60 days of age, but not at later timepoints) was observed when the protein restriction was limited to pregnancy (not lactation) (28, 29). And no change in food intake was observed, even with the introduction of a high fat diet, when the protein restriction was limited to mid or late gestation (30). Interestingly, when calorie restriction during pregnancy (as opposed to protein restriction) has been studied in the rat, an increase in food intake appears to be a more robust finding (31, 32). A survey of the literature found no studies that examined the effect of protein restriction on offspring locomotor activity. Undernutrition during pregnancy in the rat coupled with high fat diet at weaning was found to increase sedentary behavior (33), a pattern which is opposite to what we observed in the mouse, however a direct comparison between the two studies is difficult given the difference in species (mouse versus rat), model (low protein versus reduced caloric intake), timeframe (pregnancy and lactation versus gestation only) and hypercaloric diet (60% versus 30% high fat diet).
The glucose tolerance test revealed that males, but not females, on a control diet from the low protein pregnancies had significantly increased fasting glucose levels at the start of the challenge as compared to control offspring, which may indicate an increased risk for the development of diabetes. Given that females were studied later than males (6 months versus 3 months), if anything a more severe, not less severe, phenotype may have been expected in the females. Similar reports in the literature have been inconsistent (11, 13), with fasting glucose levels either unchanged in males and females (7), increased only in females (8), or increased in males (6), while AUC of the GTT was not elevated (7, 8) or increased in males only (6).
In summary, these data demonstrate that early life protein restriction can be used as a mouse model of low birth weight offspring with associated metabolic alterations. Consistent with other reports that have used protein restriction through both pregnancy and lactation (5, 13, 26), both males and females maintain a lower bodyweight regardless of the postweaning diet. LP males show a difference in metabolic parameters only when maintained on the high fat diet from weaning, while LP females showed increased food intkae and were hypometabolic, regardless of the postweaning diet, indicating an increased sensitivity of the female offspring to the in utero insult. This increased sensitivity may lead to a greater risk for obesity and/or increased adiposity over time, particularly with the loss of estrogen in later life (34). The present findings in combination with the extensive related literature highlight the importance of key variables (species, timing, model, sex, postnatal diet) that affect the in utero programming of metabolic endpoints. The present studies significantly enhance our understanding of the metabolic effects associated with low birth weight by defining how metabolic adaptations are altered by postweaning diet and offspring sex in a mouse model.
Supplementary Material
Acknowledgments
Funding Sources. The current work was supported by NIH DK064086 (Reyes) and MH087978 (Reyes). The funding source(s) had no involvement in study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest. The authors state no conflicts of interest.
References
- 1.Barker DJ. The fetal origins of diseases of old age. Eur J Clin Nutr. 1992;46(Suppl 3):S3–S9. [PubMed] [Google Scholar]
- 2.Rosebloom TJ, Meulen JHvd, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol. 2001;185(1–2):93–98. doi: 10.1016/s0303-7207(01)00721-3. [DOI] [PubMed] [Google Scholar]
- 3.Yang Z, Zhao W, Zhang X, Mu R, Zhai Y, Kong L, et al. Impact of famine during pregnancy and infancy on health in adulthood. Obes Rev. 2008;9(Suppl 1):95–99. doi: 10.1111/j.1467-789X.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- 4.Li Y, He Y, Qi L, Jaddoe VW, Feskens EJ, Yang X, et al. Exposure to the Chinese famine in early life and the risk of hyperglycemia and type 2 diabetes in adulthood. Diabetes. 2010;59(10):2400–2406. doi: 10.2337/db10-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bieswal F, Ahn MT, Reusens B, Holvoet P, Raes M, Rees WD, et al. The importance of catch-up growth after early malnutrition for the programming of obesity in male rat. Obesity (Silver Spring) 2006;14(8):1330–1343. doi: 10.1038/oby.2006.151. [DOI] [PubMed] [Google Scholar]
- 6.Ozanne SE, Olsen GS, Hansen LL, Tingey KJ, Nave BT, Wang CL, et al. Early growth restriction leads to down regulation of protein kinase C zeta and insulin resistance in skeletal muscle. J Endocrinol. 2003;177(2):235–241. doi: 10.1677/joe.0.1770235. [DOI] [PubMed] [Google Scholar]
- 7.Zambrano E, Bautista CJ, Deas M, Martinez-Samayoa PM, Gonzalez-Zamorano M, Ledesma H, et al. A low maternal protein diet during pregnancy and lactation has sex- and window of exposure-specific effects on offspring growth and food intake, glucose metabolism and serum leptin in the rat. J Physiol. 2006;571(Pt 1):221–230. doi: 10.1113/jphysiol.2005.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugden MC, Holness MJ. Gender-specific programming of insulin secretion and action. J Endocrinol. 2002;175(3):757–767. doi: 10.1677/joe.0.1750757. [DOI] [PubMed] [Google Scholar]
- 9.Stocker CJ, Arch JR, Cawthorne MA. Fetal origins of insulin resistance and obesity. Proc Nutr Soc. 2005;64(2):143–151. doi: 10.1079/pns2005417. [DOI] [PubMed] [Google Scholar]
- 10.Bol VV, Delattre AI, Reusens B, Raes M, Remacle C. Forced catch-up growth after fetal protein restriction alters the adipose tissue gene expression program leading to obesity in adult mice. Am J Physiol Regul Integr Comp Physiol. 2009;297(2):R291–R299. doi: 10.1152/ajpregu.90497.2008. [DOI] [PubMed] [Google Scholar]
- 11.Sutton GM, Centanni AV, Butler AA. Protein malnutrition during pregnancy in C57BL/6J mice results in offspring with altered circadian physiology before obesity. Endocrinology. 2010;151(4):1570–1580. doi: 10.1210/en.2009-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sellayah D, Sek K, Anthony FW, Watkins AJ, Osmond C, Fleming TP, et al. Appetite regulatory mechanisms and food intake in mice are sensitive to mismatch in diets between pregnancy and postnatal periods. Brain Res. 2008;1237:146–152. doi: 10.1016/j.brainres.2008.07.126. [DOI] [PubMed] [Google Scholar]
- 13.Bhasin KK, Nas Av, Martin LJ, Davis RC, Devaskar SU, Lusis AJ. Maternal low-protein diet or hypercholesterolemia reduces circulating essential amino acids and leads to intrauterine growth restriction. Diabetes. 2009;58(3):559–566. doi: 10.2337/db07-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellinger L, Lilley C, SC, SCL-E Prenatal exposure to a maternal low-protein diet programmes a preference for high-fat foods in the young adult rat. Br J Nutr. 2004;92(3):513–520. doi: 10.1079/bjn20041224. [DOI] [PubMed] [Google Scholar]
- 15.Tonkiss J, Almeida SS, Galler JR. Prenatally malnourished female but not male rats show increased sensitivity to MK-801 in a differential reinforcement of low rates task. Behav Pharmacol. 1998;9(1):49–60. [PubMed] [Google Scholar]
- 16.Desai M, Babu J, Ross MG. Programmed metabolic syndrome: prenatal undernutrition and postweaning overnutrition. Am J Physiol Regul Integr Comp Physiol. 2007;293(6):R2306–R2314. doi: 10.1152/ajpregu.00783.2006. [DOI] [PubMed] [Google Scholar]
- 17.Sardinha FL, Telles MM, Albuquerque KT, Oyama LM, Guimarães PA, Santos OF, et al. Gender difference in the effect of intrauterine malnutrition on the central anorexigenic action of insulin in adult rats. Nutrition. 2006;22(11–12):1152–1161. doi: 10.1016/j.nut.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Vucetic Z, Totoki K, Schoch H, Whitaker KW, Hill-Smith T, Lucki I, et al. Early life protein restriction alters dopamine circuitry. Neuroscience. 2010;168(2):359–370. doi: 10.1016/j.neuroscience.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lahti J, Räikkönen K, Kajantie E, Heinonen K, Pesonen AK, Järvenpää AL, et al. Small body size at birth and behavioural symptoms of ADHD in children aged five to six years. J Child Psychol Psychiatry. 2006;47(11):1167–1174. doi: 10.1111/j.1469-7610.2006.01661.x. [DOI] [PubMed] [Google Scholar]
- 20.Mick E, Biederman J, Prince J, Fischer MJ, Faraone SV. Impact of low birth weight on attention-deficit hyperactivity disorder. J Dev Behav Pediatr. 2002;23(1):16–22. doi: 10.1097/00004703-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Waring ME, Lapane KL. Overweight in children and adolescents in relation to attention-deficit/hyperactivity disorder: results from a national sample. Pediatrics. 2008;122(1):e1–e6. doi: 10.1542/peds.2007-1955. [DOI] [PubMed] [Google Scholar]
- 22.Shi H, Seeley RJ, Clegg DJ. Sexual differences in the control of energy homeostasis. Front Neuroendocrinol. 2009;30(3):396–404. doi: 10.1016/j.yfrne.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ozanne SE, Hales CN. Early programming of glucose-insulin metabolism. Trends Endocrinol Metab. 2002;13(9):368–373. doi: 10.1016/s1043-2760(02)00666-5. [DOI] [PubMed] [Google Scholar]
- 24.Ozanne SE, Lewis R, Jennings BJ, Hales CN. Early programming of weight gain in mice prevents the induction of obesity by a highly palatable diet. Clin Sci (Lond) 2004;106(2):141–145. doi: 10.1042/CS20030278. [DOI] [PubMed] [Google Scholar]
- 25.Ozanne SE, Nave BT, Wang CL, Shepherd PR, Prins J, Smith GD. Poor fetal nutrition causes long-term changes in expression of insulin signaling components in adipocytes. Am J Physiol. 1997;273(Pt 1):E46–E51. doi: 10.1152/ajpendo.1997.273.1.E46. [DOI] [PubMed] [Google Scholar]
- 26.Chen JH, Martin-Gronert MS, Tarry-Adkins J, Ozanne SE. Maternal protein restriction affects postnatal growth and the expression of key proteins involved in lifespan regulation in mice. PLoS One. 2009;4(3):e4950. doi: 10.1371/journal.pone.0004950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Augustyniak RA, Singh K, Zeldes D, Singh M, Rossi NF. Maternal protein restriction leads to hyperresponsiveness to stress and salt-sensitive hypertension in male offspring. Am J Physiol Regul Integr Comp Physiol. 2010;298(5):R1375–R1382. doi: 10.1152/ajpregu.00848.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coupé B, Grit I, Darmaun D, Parnet P. The timing of "catch-up growth" affects metabolism and appetite regulation in male rats born with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R813–R824. doi: 10.1152/ajpregu.00201.2009. [DOI] [PubMed] [Google Scholar]
- 29.Orozco-Sólis R, Souza SLd, Matos RJB, Grit I, Bloch JL, Nguyen P, et al. Perinatal undernutrition-induced obesity is independent of the developmental programming of feeding. Physiol Behav. 2009;96(3):481–492. doi: 10.1016/j.physbeh.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 30.Erhuma A, Bellinger L, Langley-Evans SC, Bennett AJ. Prenatal exposure to undernutrition and programming of responses to high-fat feeding in the rat. Br J Nutr. 2007;98(3):517–524. doi: 10.1017/S0007114507721505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desai M, Gayle D, Babu J, Ross MG. Programmed obesity in intrauterine growth-restricted newborns: modulation by newborn nutrition. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R91–R96. doi: 10.1152/ajpregu.00340.2004. [DOI] [PubMed] [Google Scholar]
- 32.Delahaye F, Lukaszewski MA, Wattez JS, Cisse O, Dutriez-Casteloot I, Fajardy I, et al. Maternal perinatal undernutrition programs a "brown-like" phenotype of gonadal white fat in male rat at weaning. Am J Physiol Regul Integr Comp Physiol. 2010;299(1):R101–R110. doi: 10.1152/ajpregu.00604.2009. [DOI] [PubMed] [Google Scholar]
- 33.Vickers MH, Breier BH, McCarthy D, Gluckman PD. Sedentary behavior during postnatal life is determined by the prenatal environment and exacerbated by postnatal hypercaloric nutrition. Am J Physiol Regul Integr Comp Physiol. 2003;285(1):R271–R273. doi: 10.1152/ajpregu.00051.2003. [DOI] [PubMed] [Google Scholar]
- 34.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55(4):978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.