Abstract
Background
Dynamization involves a reduction in fixation construct stiffness during bone healing, allowing increased interfragmentary movement of the fracture through physiologic weightbearing and muscle contraction. Within some optimal range, interfragmentary movement stimulates healing, but this range likely varies across stages of bone healing.
Questions/purposes
How does the time of dynamization affect the cartilage formation, bony bridging, and bone resorption in a rat fracture-healing model?
Methods
Unilateral external fixators, stabilizing a 1-mm gap, were dynamized at 1 (D1 group, n = 10), 3 (D3 group, n = 11), or 4 (D4 group, n = 11) weeks postoperatively. Continuously 5 weeks stiff (S group, n = 10) and flexible (F group, n = 11) fixation were included for comparison. After 5 weeks, healing was evaluated by histomorphometric methods.
Results
Advanced healing, indicated by less cartilage and a greater rate of bony bridging, was observed in the S group compared to the D1 or F group. In contrast, the D3 and D4 groups had less cartilage and more bridging compared to the S group. Also, the S group had less cortical resorption than the F and D1 groups.
Conclusions
These data suggest late dynamization at the onset of bony bridging led to enhanced healing, whereas dynamization at the early stage of cartilage differentiation delayed healing.
Clinical Relevance
Although our observations from this small-animal study cannot be directly transferred to humans, these data suggest, once bony bridging begins, dynamization may stimulate bone healing and accelerate remodeling.
Introduction
Mechanical factors regulate bone healing when a sufficient blood supply exists [27]. Clinically, the mechanical environment can be altered through increasing the amount of interfragmentary movement (IFM) by reducing the fixation stiffness of interlocked nails [2, 4, 38–42] or external fixators [12, 17, 28]. The IFM is also influenced by the load on the operated leg due to gravity and muscle forces [7, 14, 20, 36, 43]. For a similar loading condition, the fixation stiffness is the dominating factor for the IFM [14]. IFM reportedly is not markedly altered during various different loading conditions [14], although the stiffness of the fixation correlates with the IFM [13].
Several studies in small [26] and large [15, 16] animals suggest decreased fixation stiffness results in decreased flexural or torsional rigidity of the healing bone. Histologically, larger IFM leads to the predominance of endochondral ossification with a prolonged chondral phase and later bone formation [16, 22, 23, 25, 36, 43] and may cause bone resorption at the fragment ends [31]. The bridging of the periosteal and intracortical fracture gaps and restoration of the marrow canal indicate the final healing phase [27].
The recent development of well-controlled external fixation systems for the rat allows exploration of how mechanical stimulation in terms of the IFM can temporally influence the healing process [7, 24, 29, 30, 32, 37].
We previously found early dynamization leads to lower flexural rigidity of the healing bone [7] while late dynamization leads to flexural rigidity similar to continuously stiff fixation. However, these observations did not document how the tissue differentiation in the healing callus is affected by the time of dynamization. In particular, the tissue composition of fibrous tissue and cartilage, as well as possible bone resorption processes due to high interfragmentary strain (IFS) [31], could not be described so far. However, the knowledge of tissue composition would allow a better insight into the process of bone healing.
Several studies report the effect of later dynamization in the healing process. Utvag et al. [34] demonstrated dynamization of a rat femoral fracture model after 20 days of healing resulted in greater callus formation and better mechanical properties and bone mineral content in the healed bones at 6 weeks postfracture, compared to continuously stiff fixation. Replacing a stiff with a flexible nail reportedly did not enhance healing [33]. A possible explanation is the disturbance of the early callus formation by the reoperation.
To clarify the effect of the time of dynamization on the tissue differentiation, we tested the following hypotheses: (1) a stiff fixation would lead to less cartilage formation than a flexible fixation or early dynamization but more cartilage compared to late dynamization; (2) a stiff fixation would enhance bony bridging of periosteal and intracortical gaps compared to a flexible fixation or early dynamization but delay bridging compared to late dynamization; and (3) a stiff fixation would lead to less cortical bone resorption than a flexible fixation and early dynamization.
Materials and Methods
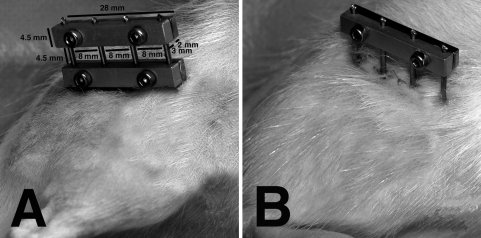
We divided 53 male Wistar rats (weight at operation, 414 ± 22 g) into five groups: stiff fixation (S group: n = 10), flexible fixation (F group: n = 11), dynamized at 1 week (D1 group: n = 10) [7], dynamized at 3 weeks (D3 group: n = 11), and dynamized at 4 weeks (D4 group: n = 11). The S and F groups had a stiff or a flexible fixation, respectively, for the entire 5-week experimental period (Fig. 1). Dynamization was performed after 1, 3, and 4 weeks postoperatively by removing the inner bar of the fixator (Fig. 1). The animals were then left with a one-fixation bar configuration, with the bar set at a 15-mm offset, for the remainder of the 5-week experimental time period. The external unilateral fixator included a frontside stainless steel and backside aluminum fixator bar (28 × 4.5 × 5 mm). Four titanium threaded pins (0.65-mm core diameter/1.2-mm outer diameter), spaced 8 mm apart, were held between the two sides of the fixator bar under compression. The stiff fixation utilized two fixator bars, one set at a 6-mm offset and the other at a 15-mm offset (Fig. 1). The more flexible fixation utilized a single fixator bar set at a 15-mm offset. The offset was defined as the free length of the pins between the lateral surface of the rat’s femur and the inner side of the fixator bar. An in vitro experiment was performed to measure the stiffness of the two fixator configurations. Polymer tubes, used as a model for the femur, with an osteotomy gap of 1 mm with the applied fixators were loaded in the axial direction of the tubes. The load-deflection curves showed a stiffness of 10 N/mm for the flexible configuration and 74 N/mm for the stiff configuration. The protocol was in accordance with the principles of the Guide for the Care and Use of Laboratory Animals and was approved by the local regulatory agency (Regierungspräsidium Tübingen, Number 857).
Fig. 1A–B.
Photographs of a (A) stiff and (B) flexible fixator, with dimensions, are shown. For the two-fixator bar configuration, one bar was set at an offset of 6 mm between the lateral surface of the femur and the inner side of the fixator bar, while the second bar had an offset of 15 mm from the surface of the femur. The 3-mm-thick bar at the front was made of stainless steel whereas the 2-mm-thick component behind was made of aluminum.
Sample size calculations were originally based on the expected differences (and SDs) between the rigid and the flexible fixation from results of a similar study by Mark et al. [24], with an effect size of 30% (SD = 25%). These calculations assumed the Type I error rate would be controlled at 5% and the desired power of 80%. The resulting sample size calculations suggested 12 rats would likely provide sufficient power for the end point of bending rigidity data, which was the initial outcome performed on the samples before histologic analysis [7].
The operative procedure has been previously reported [7]; in brief, rats were injected subcutaneously with atropine sulfate (0.05 mg/kg), followed by an intraperitoneal injection of a solution containing ketamine hydrochloride (70 mg/kg) and xylaline hydrochloride (9 mg/kg). The antibiotic clindamycin-2-dihydrogenphosphate (45 mg/kg) was also administered subcutaneously. An incision was made across the lateral aspect of the thigh, through the fascia, exposing the femur by separating the gluteus superficialis and biceps femoris muscles. Four threaded titanium pins were manually screwed into the femur after predrilling with a 0.9-mm drill bit resulting in a firm fixation. The external fixator was attached, allowing either a 6- or 15-mm offset. A 1-mm osteotomy gap was created in the middle of the femur using a circular saw, after which time the fascia and skin were sutured. The analgesic tramadol was administered subcutaneously immediately after surgery (20 mg/kg) and over 3 days diluted in the animals’ drinking water (25 mg/L). An antibiotic clindamycin-2-dihydrogenphosphate (45 mg/kg) was also administered subcutaneously 3 days postoperatively. Immediately after surgery, the rats were allowed to resume normal activity and given unrestricted access to food and water.
Most animals tolerated the experimental procedure well; however, the following complications were observed: anesthesia-associated death (n = 2), pin infection (n = 3), and pin breakage (n = 7). After exclusion of these 12 rats, the F, S, D1, and D4 groups each consisted of n = 8, while the D3 group consisted of n = 9.
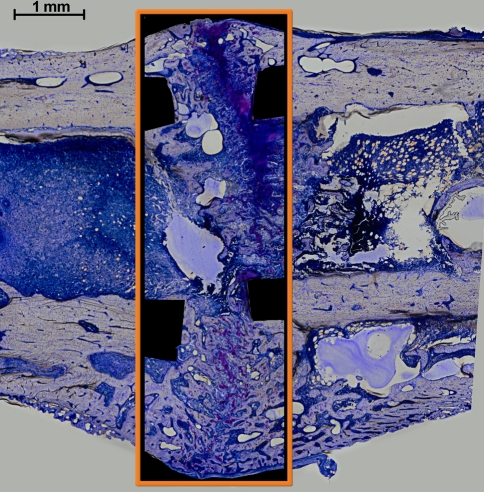
After 35 days of healing, rats were sacrificed in a carbon dioxide chamber and the femurs were dehydrated in ascending grades of ethanol, infiltrated, and embedded in methyl methacrylate, sectioned in the longitudinal direction, and stained with paragon. This stain permitted the differentiation among fibrous tissue (white and light blue), cartilage (purple), and bone (white-yellow). The morphologic features of the periosteal, intracortical, and endosteal zones were qualitatively described using light microscopy. Additionally, quantitative histomorphometry was performed using light microscopy (KS400; Zeiss, Eching, Germany) at ×200 magnification to analyze tissue differentiation for a single fixed region of interest (ROI). Histologic measurements were performed by a single experienced observer (BMW). The fixed ROI included the complete outer diameter of the periosteal callus in the radial direction and extended 2 mm proximally and distally from the center of the gap, totaling 20 mm2 (Fig. 2). The cortical bone was excluded from the ROI. The total callus area was measured (KS400) and a point-counting method was used to quantify the tissue distribution in the callus using a digital grid [9]. A high-resolution digital image was taken of the entire ROI under ×200 magnification. Using standard software (Photoshop®; Adobe Systems Inc, San Jose, CA), a grid was superimposed onto the image of the 20-mm2 ROI area. The tissue visible underneath the 1407 points of the grid was analyzed and counted. The number of points covered by each tissue type was expressed as a percentage of the total number of points (Table 1). As the individual callus areas varied, points on the grid covering empty area outside the boundaries of a particular callus were excluded from analysis. The grid-measuring method was performed in a blinded fashion and has repeatability of better than 90% [8, 9]. The number of animals achieving bony bridging of the osteotomy gap in the periosteal (medial and lateral), intracortical (medial and lateral), and endosteal zones was quantified (Table 2). A bridging score from 0 to 4 was calculated based on the number of cortices (medial periosteal, lateral periosteal, medial intracortical, and lateral intracortical) resulting in bony bridging. Unlike the periosteal or intracortical bridging that indicates advanced remodeling, endosteal bridging does not reflect advanced healing. Lack of endosteal bridging and resorption of the endosteal callus with its reconstitution by bone marrow are signs of more advanced healing. A cortical bone resorption score from 0 to 4 was also calculated based on the number of cortices (distal medial, distal lateral, proximal medial, and proximal lateral) showing signs of bone resorption at the periosteal, endosteal, or intracortical margins.
Fig. 2.
A region of interest investigated in the study is shown (within the box) (stained with paragon). Note the cortical bone fragments were excluded from analysis.
Table 1.
Total callus area and percentages of bone, cartilage, and fibrous connective tissue composing callus tissue after 5 weeks of fixation
| Region/parameter | S group | F group | D1 group | D3 group | D4 group |
|---|---|---|---|---|---|
| Total callus | |||||
| Total area (mm2) | 11.4 ± 1.0 | 12.2 ± 1.4 | 13.8 ± 1.5 | 10.1 ± 2.2 | 10.3 ± 1.5 |
| Periosteal callus (%) | 45.3 ± 10.5 | 52.7 ± 10.2 | 55.0 ± 7.1 | 33.9 ± 14.4 | 35.0 ± 15.3 |
| Intracortical callus (%) | 20.7 ± 6.0 | 21.5 ± 3.9 | 19.0 ± 7.4 | 19.9 ± 4.3 | 23.8 ± 6.3 |
| Endosteal callus (%) | 34.0 ± 9.3 | 25.8 ± 10.7 | 26.0 ± 4.4 | 46.2 ± 14.5 | 41.2 ± 15.3 |
| Bone area (%) | 40.2 ± 5.6 | 35.4 ± 10.3 | 24.1 ± 7.8 | 31.9 ± 6.3 | 30.9 ± 12.9 |
| Fibrous connective tissue area (%) | 42.4 ± 9.9 | 32.7 ± 7.7 | 34.3 ± 12.7 | 58.5 ± 12.2 | 59.0 ± 18.4 |
| Cartilage area (%) | 17.4 ± 8.1 | 31.9 ± 16.2 | 41.7 ± 19.0 | 9.7 ± 9.3 | 10.1 ± 6.8 |
| Periosteal callus | |||||
| Bone area (%) | 46.3 ± 5.1 | 36.7 ± 8.6 | 26.9 ± 9.8 | 56.1 ± 17.7 | 48.9 ± 10.7 |
| Fibrous connective tissue area (%) | 34.2 ± 7.0 | 31.3 ± 6.8 | 31.5 ± 8.9 | 31.2 ± 13.7 | 38.6 ± 12.4 |
| Cartilage area (%) | 19.5 ± 8.2 | 32.0 ± 12.9 | 41.6 ± 16.4 | 12.7 ± 9.0 | 12.5 ± 7.2 |
| Intracortical callus | |||||
| Bone area (%) | 41.0 ± 7.2 | 34.5 ± 15.3 | 17.3 ± 9.0 | 43.2 ± 16.3 | 33.7 ± 12.8 |
| Fibrous connective tissue area (%) | 37.3 ± 14.3 | 28.3 ± 13.6 | 37.4 ± 17.5 | 42.4 ± 11.4 | 53.7 ± 14.4 |
| Cartilage area (%) | 21.7 ± 13.3 | 37.2 ± 23.5 | 45.3 ± 23.9 | 14.4 ± 12.3 | 12.6 ± 8.9 |
| Endosteal callus | |||||
| Bone area (%) | 31.7 ± 8.8 | 31.9 ± 16.7 | 24.5 ± 13.4 | 13.7 ± 8.6 | 20.3 ± 21.7 |
| Fibrous connective tissue area (%) | 55.7 ± 13.3 | 38.9 ± 14.5 | 36.9 ± 19.9 | 80.3 ± 17.8 | 71.5 ± 28.6 |
| Cartilage area (%) | 12.6 ± 9.0 | 29.2 ± 24.4 | 38.6 ± 26.0 | 6.0 ± 10.4 | 8.2 ± 8.0 |
Values are expressed as mean ± SD; S group = continuously stiff fixation; F group = flexible fixation; D1 group = dynamization at 1 week; D3 group = dynamization at 3 weeks; D4 group = dynamization at 4 weeks.
Table 2.
Number of animals achieving bony bridging or exhibiting cortical bone resorption after 5 weeks of healing for each region of the callus
| Region | S group | F group | D1 group | D3 group | D4 group |
|---|---|---|---|---|---|
| Periosteal callus bridging | |||||
| Medial | 3/8 | 2/8 | 3/8 | 9/9 | 8/8 |
| Lateral | 3/8 | 0/8 | 1/8 | 7/9 | 6/8 |
| Intracortical callus bridging | |||||
| Medial | 4/8 | 3/8 | 1/8 | 7/9 | 8/8 |
| Lateral | 1/8 | 2/8 | 1/8 | 6/9 | 8/8 |
| Total % bridging (periosteal + intracortical), bridging score* | 34%, 1.4 ± 1.4 | 22%, 0.9 ± 1.0 | 19%, 0.8 ± 1.4 | 81%, 3.2 ± 1.2 | 94%, 3.8 ± 0.5 |
| Endosteal callus bridging | 5/8 | 3/8 | 1/8 | 2/9 | 3/8 |
| Cortical bone resorption | |||||
| Distal medial | 2/8 | 3/8 | 5/8 | 2/9 | 3/8 |
| Distal lateral | 5/8 | 6/8 | 7/8 | 3/9 | 5/8 |
| Proximal medial | 8/8 | 7/8 | 8/8 | 2/9 | 4/8 |
| Proximal lateral | 4/8 | 7/8 | 8/8 | 2/9 | 2/8 |
| Total % resorption (distal + proximal) | 59% | 72% | 88% | 25% | 44% |
* Values are expressed as mean ± SD; S group = continuously stiff fixation; F group = continuously flexible fixation; D1 group = dynamization at 1 week; D3 group = dynamization at 3 weeks; D4 group = dynamization at 4 weeks.
We determined differences in the amount of cartilage formation, the number of cortices with bony bridging, and the number of cortices with bone resorption between the continuously stiff fixation group (S group) and the other four stabilization groups (F, D1, D3, and D4 groups) using either an independent-group t test or a Mann-Whitney U test, depending on normality, determined by a Shapiro-Wilk test. All p values were adjusted for the four multiple comparisons, using the Hochberg procedure [21]. Analyses were performed using standard statistical software (SAS® 9.1; SAS Institute Inc, Cary, NC).
Results
The amount of cartilage was less (p = 0.020) in the S group than in D1 group (Fig. 3) and tended to be less than in the F group (Table 1). There was a trend of a greater amount of cartilage in the S group compared to the D3 and D4 groups (Fig. 4).
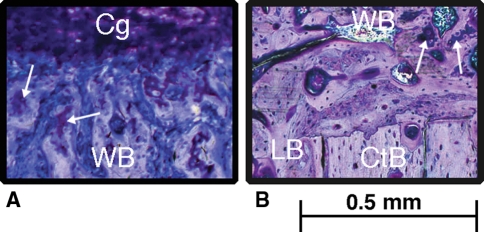
Fig. 3A–B.
High-magnification histologic images from the (A) F group and (B) D3 group show the distribution of tissue (WB = woven bone [pink-white]; Cg = cartilage [purple]; LB = lamellar bone [pink-white]; CtB = cortical bone [pink-white]) within the fracture callus (stained with paragon). Islands of calcified cartilage (arrows) were also observed.
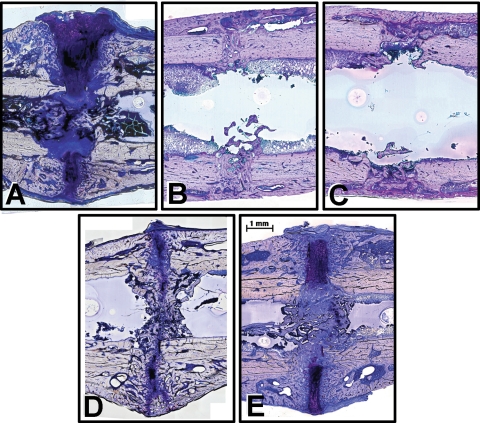
Fig. 4A–E.
Histologic images show the callus after 5 weeks of healing in the (A) D1 group, (B) D3 group, (C) D4 group, (D) S group, and (E) F group. Histologic sections were stained with paragon: fibrous tissue (white and blue), cartilage (purple), and bone (pink-white).
The bridging score data demonstrated a trend of more bridged cortices in the S group (34%) than in the F group (22%) and D1 group (19%) and less than in the D3 group (81%, p = 0.032) and D4 group (94%, p = 0.002) (Table 2). The D4 group was the only group showing a complete bridging of all medial and lateral periosteal and intracortical regions.
The S group had a lower number of cortices that exhibited resorption (59%) compared to the D1 group (88%, p = 0.029) and the F group (Table 2). In contrast, the S group had a greater number of cortices that exhibited resorption compared to the D3 group (p = 0.010) and D4 group (Fig. 5).
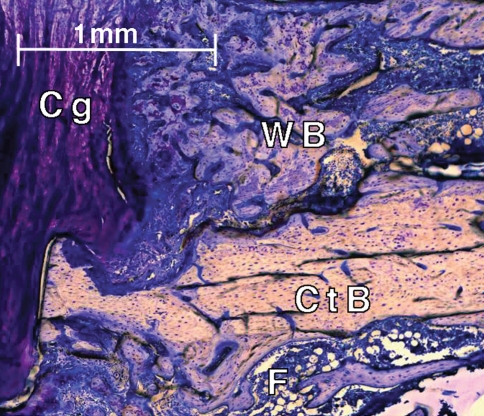
Fig. 5.
An histologic image shows resorption of the cortical bone in the D1 group after 5 weeks of healing. Histologic sections were stained with paragon: fibrous tissue (F, white and blue), cartilage (Cg, purple), and bone, including woven bone (WB) and cortical bone (CtB) (pink-white).
Although not part of our original hypotheses, endosteal bony bridging was unexpectedly most often observed in the S group (63%) (Table 2). In contrast, the late dynamized groups (D3: 22%; D4: 38%) had few animals with endosteal bridging and the endosteal callus was almost completely resorbed, resulting in an intramedullary space that was mainly reconstituted with bone marrow (Fig. 4). The F group (38%) and D1 group (13%) also had few animals with endosteal bridging, but in both cases the endosteal canal was filled with cartilage and fibrous connective tissue rather than the marrow-related fibrous tissue observed in the D3 and D4 groups. The large proportion of marrow-related fibrous tissue compared to bone and cartilage observed in the endosteal region of the D3 and D4 groups further demonstrated advanced healing and remodeling in these groups (Table 1).
Discussion
Since the optimal time for dynamization of a fracture is still unknown, we evaluated the effect of dynamization at early and later stages of bone healing on the cartilage formation, bony bridging, and bone resorption in a rat diaphyseal femoral osteotomy model. We therefore tested the following hypotheses: (1) a stiff fixation would lead to less cartilage formation than a flexible fixation or early dynamization but more cartilage compared to late dynamization; (2) a stiff fixation would enhance bony bridging of periosteal and intracortical gaps compared to a flexible fixation or early dynamization but delay bridging compared to late dynamization; and (3) a stiff fixation would lead to less cortical bone resorption than a flexible fixation and early dynamization.
We recognize limitations to our experiment. First, the study was performed in a small-animal model. Although our results cannot directly be transferred to humans, previous experimental data from a large-animal model [1] correlated with our results showing early loading and IFM initiated a large amount of periosteal callus and cartilage but delayed healing. In principle, the effect of IFM on callus formation is the same in the small- and large-animal models, as well as the human situation, although the time course of healing is much faster in the small-animal model. Independent of the time course, it can be concluded the late phase of healing would be better for dynamization. Second, there are no clear definitions or guidelines for deciding whether fixation is stiff or flexible and the resulting IFS guiding the tissue differentiation can only be estimated in rats. When the experiment was designed, the stiffness of the fixation and the subsequent IFM and IFS were estimated based on load bearing of the rat [11] and the ratio of load bearing and femoral loading in quadrupeds [3]. Taking into consideration that the rats loaded their leg postoperatively approximately 50% (unpublished data from our laboratory) of the preoperative level, we calculated an IFS of 32% for the stiff group and 100% for the flexible group. Whereas 32% is a stable fixation that provides enough IFS to stimulate callus formation, 100% (contact between the fragments) is a very flexible fixation. Recent data demonstrated the loading in the rat femur could even be higher [35]. Clinically, the risk of a too flexible fixation generally outweighs the risk of a too stiff fixation [14]. Third, we examined the influence of only two external fixation stiffnesses (one rigid and one flexible) on healing. However, the flexible fixator stiffness we chose was comparable to that reported for ring fixators used clinically [14], and the rigid fixator stiffness was similar to an external fixator also used clinically [18, 20].
Our observations support the first hypothesis that the stiff fixation (S group) would lead to less cartilage formation than the flexible group (F group) and group dynamized at 1 week (D1 group) but more than that with the groups dynamized at 3 or 4 weeks (D3 or D4 groups). Our histologic data from the rat concur with studies in large-animal models [1, 16, 22, 23] and small-animal models [25, 36, 43] also showing a prolonged chondral phase and delayed healing resulted from increased IFM during the early phase of healing. It has been proposed early higher-magnitude mechanical stimulation may influence stem cell proliferation and promote differentiation into a cartilage phenotype, thereby altering regenerative processes [5, 19]. The late-dynamized groups both had a smaller total callus area and a smaller proportion of cartilage in the callus compared to the continuously stiff fixation group. Our data agree with the results from a previous study [34], which dynamized by unlocking an interlocking nail after 20 days of healing. Similar to their data for statically locked nails, late dynamization resulted in improved biomechanical properties and mineralization compared to continuously stiff fixation. Another study by the same group [33], where a stiff nail was replaced by a flexible nail, could not demonstrate an advantage of this dynamization. The reason might be this reoperation destroyed the young callus and caused a delay in healing.
Our second hypothesis tested whether the S group would have more bridging than the F and D1 groups but less than the D3 or D4 groups. However, we observed only a nonsignificant trend toward a lower percentage of bony bridging in the F and D1 groups than in the S group. A greater number of animals from the D3 and D4 groups achieved bony bridging after 5 weeks of healing compared to the S group. The reason could be, in the later phase of healing, the existing callus had already reduced the possible IFM to such an extent that the higher loading by dynamization did not lead to critical IFS. Such a rapid reduction of IFM with healing time was demonstrated in sheep studies [10]. The low rate of endosteal bony bridging observed in the D3 and D4 groups was a sign of advanced bone healing and remodeling, as the endosteal callus was almost completely resorbed and the resulting intramedullary space in these groups was already mainly reconstituted with bone marrow. These data indicate late dynamization at the onset of bony bridging enhanced healing as evidenced by the advanced bone remodeling.
Our observations confirmed the S group tended to have less cortical bone resorption than the F group and less cortical bone resorption compared to the D1 group. High IFS as estimated in the F and D1 groups with contact of the bony fragments causes bone resorption, which is known from earlier studies on larger animals [31]. As mentioned previously, this might be different when the dynamization is performed late and the already existing callus is reducing the IFM and IFS to uncritical values. Taking into account that the axial forces on the rat femur are perhaps larger than estimated in our first calculation and individual differences between animals occur, it could be, even under stiffer fixation, critical IFS might occasionally occur under high loading, thus causing bone resorption in a few cases.
Our data suggest greater IFM during the early phase of healing (dynamization) is detrimental. However, with callus formation of sufficient tissue maturity, late dynamization could likely enhance uneventful healing when there is a reasonable reduction of the fragments. Early dynamization would likely only be beneficial clinically in the context that it would allow closure of the fracture gap through the fixator’s or nail’s telescoping mechanism [6].
Acknowledgments
The authors thank Dr. Anja Peters for her substantial contributions to the histologic analysis, Ursula Maile and Marion Tomo for their assistance with histologic preparation, and Dr. Christine Bausewein and John Besse for their help during the operative procedures.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Augat P, Merk J, Ignatius A, Margevicius K, Bauer G, Rosenbaum D, Claes L. Early, full weightbearing with flexible fixation delays fracture healing. Clin Orthop Relat Res. 1996;328:194–202. doi: 10.1097/00003086-199607000-00031. [DOI] [PubMed] [Google Scholar]
- 2.Basumallick MN, Bandopadhyay A. Effect of dynamization in open interlocking nailing of femoral fractures: a prospective randomized comparative study of 50 cases with a 2-year follow-up. Acta Orthop Belg. 2002;68:42–48. [PubMed] [Google Scholar]
- 3.Bergmann G, Graichen F, Rohlmann A. Hip joint forces in sheep. J Biomech. 1999;32:769–777. doi: 10.1016/S0021-9290(99)00068-8. [DOI] [PubMed] [Google Scholar]
- 4.Brumback RJ, Uwagie-Ero S, Lakatos RP, Poka A, Bathon GH, Burgess AR. Intramedullary nailing of femoral shaft fractures Part II. Fracture-healing with static interlocking fixation. J Bone Joint Surg Am. 1988;70:1453–1462. [PubMed] [Google Scholar]
- 5.Chao EY, Inoue N, Elias JJ, Aro H. Enhancement of fracture healing by mechanical and surgical intervention. Clin Orthop Relat Res. 1998;355(Suppl):S163–S178. doi: 10.1097/00003086-199810001-00018. [DOI] [PubMed] [Google Scholar]
- 6.Claes L, Augat P, Suger G, Wilke HJ. Influence of size and stability of the osteotomy gap on the success of fracture healing. J Orthop Res. 1997;15:577–584. doi: 10.1002/jor.1100150414. [DOI] [PubMed] [Google Scholar]
- 7.Claes L, Blakytny R, Gockelmann M, Schoen M, Ignatius A, Willie B. Early dynamization by reduced fixation stiffness does not improve fracture healing in a rat femoral osteotomy model. J Orthop Res. 2009;27:22–27. doi: 10.1002/jor.20712. [DOI] [PubMed] [Google Scholar]
- 8.Claes L, Maurer-Klein N, Henke T, Gerngross H, Melnyk M, Augat P. Moderate soft tissue trauma delays new bone formation only in the early phase of fracture healing. J Orthop Res. 2006;24:1178–1185. doi: 10.1002/jor.20173. [DOI] [PubMed] [Google Scholar]
- 9.Claes L, Ruter A, Mayr E. Low-intensity ultrasound enhances maturation of callus after segmental transport. Clin Orthop Relat Res. 2005;430:189–194. doi: 10.1097/01.blo.0000150456.39608.bc. [DOI] [PubMed] [Google Scholar]
- 10.Claes LE, Heigele CA, Neidlinger-Wilke C, Kaspar D, Seidl W, Margevicius KJ, Augat P. Effects of mechanical factors on the fracture healing process. Clin Orthop Relat Res. 1998;355(Suppl):S132–S147. doi: 10.1097/00003086-199810001-00015. [DOI] [PubMed] [Google Scholar]
- 11.Clarke KA. Differential fore- and hindpaw force transmission in the walking rat. Physiol Behav. 1995;58:415–419. doi: 10.1016/0031-9384(95)00072-Q. [DOI] [PubMed] [Google Scholar]
- 12.Bastiani G, Aldegheri R, Renzi Brivio L. The treatment of fractures with a dynamic axial fixator. J Bone Joint Surg Br. 1984;66:538–545. doi: 10.1302/0301-620X.66B4.6746689. [DOI] [PubMed] [Google Scholar]
- 13.Duda GN, Kirchner H, Wilke HJ, Claes L. A method to determine the 3-D stiffness of fracture fixation devices and its application to predict inter-fragmentary movement. J Biomech. 1998;31:247–252. doi: 10.1016/S0021-9290(97)00115-2. [DOI] [PubMed] [Google Scholar]
- 14.Duda GN, Sollmann M, Sporrer S, Hoffmann JE, Kassi JP, Khodadadyan C, Raschke M. Interfragmentary motion in tibial osteotomies stabilized with ring fixators. Clin Orthop Relat Res. 2002;396:163–172. doi: 10.1097/00003086-200203000-00025. [DOI] [PubMed] [Google Scholar]
- 15.Epari DR, Kassi JP, Schell H, Duda GN. Timely fracture-healing requires optimization of axial fixation stability. J Bone Joint Surg Am. 2007;89:1575–1585. doi: 10.2106/JBJS.F.00247. [DOI] [PubMed] [Google Scholar]
- 16.Epari DR, Schell H, Bail HJ, Duda GN. Instability prolongs the chondral phase during bone healing in sheep. Bone. 2006;38:864–870. doi: 10.1016/j.bone.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 17.Foxworthy M, Pringle RM. Dynamization timing and its effect on bone healing when using the Orthofix Dynamic Axial Fixator. Injury. 1995;26:117–119. doi: 10.1016/0020-1383(95)92189-H. [DOI] [PubMed] [Google Scholar]
- 18.Goodship AE, Kenwright J. The influence of induced micromovement upon the healing of experimental tibial fractures. J Bone Joint Surg Br. 1985;67:650–655. doi: 10.1302/0301-620X.67B4.4030869. [DOI] [PubMed] [Google Scholar]
- 19.Goodship AE, Lawes TJ, Rubin CT. Low-magnitude high-frequency mechanical signals accelerate and augment endochondral bone repair: preliminary evidence of efficacy. J Orthop Res. 2009;27:922–930. doi: 10.1002/jor.20824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodship AE, Watkins PE, Rigby HS, Kenwright J. The role of fixator frame stiffness in the control of fracture healing: an experimental study. J Biomech. 1993;26:1027–1035. doi: 10.1016/S0021-9290(05)80002-8. [DOI] [PubMed] [Google Scholar]
- 21.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. doi: 10.1093/biomet/75.4.800. [DOI] [Google Scholar]
- 22.Lienau J, Schell H, Duda GN, Seebeck P, Muchow S, Bail HJ. Initial vascularization and tissue differentiation are influenced by fixation stability. J Orthop Res. 2005;23:639–645. doi: 10.1016/j.orthres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Lienau J, Schell H, Epari DR, Schutze N, Jakob F, Duda GN, Bail HJ. CYR61 (CCN1) protein expression during fracture healing in an ovine tibial model and its relation to the mechanical fixation stability. J Orthop Res. 2006;24:254–262. doi: 10.1002/jor.20035. [DOI] [PubMed] [Google Scholar]
- 24.Mark H, Bergholm J, Nilsson A, Rydevik B, Stromberg L. An external fixation method and device to study fracture healing in rats. Acta Orthop Scand. 2003;74:476–482. doi: 10.1080/00016470310017820. [DOI] [PubMed] [Google Scholar]
- 25.Mark H, Nilsson A, Nannmark U, Rydevik B. Effects of fracture fixation stability on ossification in healing fractures. Clin Orthop Relat Res. 2004;419:245–250. doi: 10.1097/00003086-200402000-00040. [DOI] [PubMed] [Google Scholar]
- 26.Mark H, Rydevik B. Torsional stiffness in healing fractures: influence of ossification. An experimental study in rats. Acta Orthop. 2005;76:428–433. [PubMed] [Google Scholar]
- 27.McKibbin B. The biology of fracture healing in long bones. J Bone Joint Surg Br. 1978;60:150–162. doi: 10.1302/0301-620X.60B2.350882. [DOI] [PubMed] [Google Scholar]
- 28.Noordeen MH, Lavy CB, Shergill NS, Tuite JD, Jackson AM. Cyclical micromovement and fracture healing. J Bone Joint Surg Br. 1995;77:645–648. [PubMed] [Google Scholar]
- 29.Oest ME, Dupont KM, Kong HJ, Mooney DJ, Guldberg RE. Quantitative assessment of scaffold and growth factor-mediated repair of critically sized bone defects. J Orthop Res. 2007;25:941–950. doi: 10.1002/jor.20372. [DOI] [PubMed] [Google Scholar]
- 30.Palomares KT, Gleason RE, Mason ZD, Cullinane DM, Einhorn TA, Gerstenfeld LC, Morgan EF. Mechanical stimulation alters tissue differentiation and molecular expression during bone healing. J Orthop Res. 2009;27:1123–1132. doi: 10.1002/jor.20863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perren SM. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology. J Bone Joint Surg Br. 2002;84:1093–1110. doi: 10.1302/0301-620X.84B8.13752. [DOI] [PubMed] [Google Scholar]
- 32.Strube P, Mehta M, Putzier M, Matziolis G, Perka C, Duda GN. A new device to control mechanical environment in bone defect healing in rats. J Biomech. 2008;41:2696–2702. doi: 10.1016/j.jbiomech.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Utvag S, Korsnes L, Rindal D, Reikeras O. Influence of flexible nailing in the later phase of fracture healing: strength and mineralization in rat femora. J Orthop Sci. 2001;6:576–584. doi: 10.1007/s007760100015. [DOI] [PubMed] [Google Scholar]
- 34.Utvag S, Rindal D, Reikeras O. Effects of torsional rigidity on fracture healing: strength and mineralization in rat femora. J Orthop Trauma. 1999;13:212–219. doi: 10.1097/00005131-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Wehner T, Claes L, Niemeyer F, Nolte D, Simon U. Influence of the fixation stability on the healing—time a numerical study of a patient-specific fracture healing process. Clin Biomech (Bristol, Avon) 2010;25:606–612. doi: 10.1016/j.clinbiomech.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Williams EA, Rand JA, An KN, Chao EY, Kelly PJ. The early healing of tibial osteotomies stabilized by one-plane or two-plane external fixation. J Bone Joint Surg Am. 1987;69:355–365. [PubMed] [Google Scholar]
- 37.Willie B, Adkins K, Zheng X, Simon U, Claes L. Mechanical characterization of external fixator stiffness for a rat femoral fracture model. J Orthop Res. 2009;27:687–693. doi: 10.1002/jor.20792. [DOI] [PubMed] [Google Scholar]
- 38.Wiss DA, Brien WW, Stetson WB. Interlocked nailing for treatment of segmental fractures of the femur. J Bone Joint Surg Am. 1990;72:724–728. [PubMed] [Google Scholar]
- 39.Wiss DA, Stetson WB. Unstable fractures of the tibia treated with a reamed intramedullary interlocking nail. Clin Orthop Relat Res. 1995;315:56–63. [PubMed] [Google Scholar]
- 40.Wu CC. The effect of dynamization on slowing the healing of femur shaft fractures after interlocking nailing. J Trauma. 1997;43:263–267. doi: 10.1097/00005373-199708000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Wu CC, Chen WJ. Healing of 56 segmental femoral shaft fractures after locked nailing: poor results of dynamization. Acta Orthop Scand. 1997;68:537–540. doi: 10.3109/17453679708999022. [DOI] [PubMed] [Google Scholar]
- 42.Wu CC, Shih CH. Effect of dynamization of a static interlocking nail on fracture healing. Can J Surg. 1993;36:302–306. [PubMed] [Google Scholar]
- 43.Wu JJ, Shyr HS, Chao EY, Kelly PJ. Comparison of osteotomy healing under external fixation devices with different stiffness characteristics. J Bone Joint Surg Am. 1984;66:1258–1264. [PubMed] [Google Scholar]