Abstract
Carisoprodol is a centrally acting drug used to relieve skeletal muscle spasms and associated pain in acute musculoskeletal conditions. There is evidence from different sources that this oral muscle relaxant is abused and that it is associated with impairment leading to arrests for “driving under the influence” as well as increased risk of automobile accidents. Its subjective and psychomotor effects in healthy volunteers at therapeutic and supratherapeutic doses have not been well-characterized, and form the basis of this report. Fifteen healthy volunteers (8 males, 7 females) were administered 0, 350, and 700 mg of carisoprodol in separate sessions and for 6 h afterwards they completed a battery of tests at fixed time intervals so as to assess the subjective and psychomotor effects of the drug. The supratherapeutic dose, 700 mg, increased visual analog scale ratings of terms that were more reflective of sedation (e.g., “sleepy,” “heavy, sluggish feeling”) than those of abuse liability, and produced impaired performance on several psychomotor tests. The therapeutic dose, 350 mg, while producing few and mild subjective effects, still produced psychomotor impairment. The fact that the therapeutic dose of carisoprodol produced minimal subjective effects while adversely affecting performance is of concern in that patients prescribed this drug may feel relatively normal and engage in tasks (driving) that could put themselves and others at risk.
Keywords: carisoprodol, musculoskeletal relaxant, subjective effects, abuse liability, psychomotor performance, healthy volunteer
1. Introduction
Carisoprodol (SOMA®) is a musculoskeletal relaxant that is typically prescribed for acute back pain syndromes that involve muscle spasm (Dillon et al., 2004; Toth and Urtis, 2004). It is available in dosage formulations of 250 and 350 mg and is usually prescribed for administration three times daily and at night. Its metabolites are hydroxycarisoprodol, hydroxymeprobamate, and meprobamate, with meprobamate being the primary active metabolite (Douglas et al., 1962; Olsen et al., 1994; Dalen et al., 1996). It was once thought that the sedative properties of carisoprodol were due primarily to meprobamate, a sedative-hypnotic with barbiturate-like features (Gonzalez et al., 2009). However, signs of carisoprodol toxicity from an overdose occur before it is significantly dealkylated to meprobamate, and the signs do not resemble classic signs of meprobamate toxicity (Roth et al., 1998; Bramness et al., 2004). In fact, a recent case report suggested that the signs of carisoprodol toxicity resemble central serotonin syndrome (Bramness et al., 2005a). Whether serotonin plays a role in mediating the effects of carisoprodol remains to be determined, but there is in vitro and in vivo evidence that carisoprodol acts via the GABAergic system, independent of meprobamate. In an in vitro study, carisoprodol allosterically modulated and directly activated GABAA receptors with an efficacy and potency greater than that of meprobamate (Gonzalez et al., 2009). Preclinical drug discrimination studies demonstrate that carisoprodol has GABAergic activity in that chlordiazepoxide, pentobarbital, and meprobamate substituted for carisoprodol, and a GABA antagonist, bemegride, blocked its discriminative stimulus effects (Gonzalez et al., 2009). Given that benzodiazepines, barbiturates, and meprobamate have been abused by humans, and that carisoprodol shares discriminative stimulus properties with these drugs, it would stand to reason that carisoprodol too would be abused, and indeed this is the case. According to the National Survey on Drug Use and Health, an estimated 2.9 million people in 2009 in the US reported lifetime use of carisoprodol for non-medical purposes (Substance Abuse and Mental Health Services Administration, 2010a). An epidemiological database tracking emergency department visits related to abuse of drugs revealed that the majority of visits associated with musculoskeletal relaxants were due to carisoprodol (e.g., 29,980 visits out of a total of 49,241 visits for all skeletal muscle relaxants) (Substance Abuse and Mental Health Services Administration, 2010b). Furthermore, there are numerous case reports in the literature of carisoprodol abuse (Dougherty, 1995; Reeves et al., 1997; Venugopal et al., 2000; Gorman et al., 2005; Eleid et al., 2010). Although not scheduled by the federal Drug Enforcement Administration in the US, it is a schedule IV controlled substance in several states (Reeves and Burke, 2008, 2010). There have been calls for the federal DEA to schedule this drug or to withdraw it from the pharmacopeia (Dougherty, 1995; Reeves et al., 1999; Bramness and Skurtveit, 2008b; Reeves and Burke, 2008; Fass, 2010). Indeed, in at least one country, Norway, the drug has been withdrawn from their national pharmacopeia, not only because of its abuse but also for safety concerns (Hoiseth et al., 2009), and the European Medicines Association in 2007 recommended that all European Union countries delete carisoprodol products from their formularies (Bramness et al., 2008a). People have been charged with driving under the influence (DUI), and have displayed signs of intoxication similar to alcohol and other CNS-depressant drugs, while taking carisoprodol (Robertson and Marinetti, 2003). In some of the DUI cases, additional drugs known to produce impairment were found in toxicology testing (Logan et al., 2000), but in other cases, the only drug detected in the blood was carisoprodol (Logan et al., 2000; Bramness et al., 2004). Perhaps not surprisingly, the use of carisoprodol is associated with an increased risk of traffic accidents (Bramness et al., 2007).
We are aware of only one abuse liability assessment study conducted with carisoprodol. In that study conducted approximately 50 years ago, 15 former opiate addicts were tested with acute doses of carisoprodol ranging from 1050–2500 mg (Fraser et al., 1961a). A questionnaire was used that assessed strength of drug effect, desire to take the drug daily, and what class the drug came from (as perceived by the participants). At the 2500 mg dose, 11 subjects reported the drug either as a barbiturate or that it made them sleepy (with some subjects, it was reported that they were “profoundly” sleepy and difficult to arouse 1–2 h after drug administration). Although not explicitly stated in the manuscript, it appears that no subjects reported wanting to take the drug on a daily basis, and the authors concluded that the drug had no “addictive properties of an opiate type.” However, it is possible that the drug would have shown signs of abuse liability had sedative abusers been tested. At least four psychophamacology studies with carisoprodol have been done in non-drug-abusing volunteers but abuse liability-related measures were not used in the studies. In one study that tested placebo and 525 mg carisoprodol, visual analog scale ratings of sleepiness increased relative to placebo (Flaten et al., 1999). In a study that compared effects of 700 mg carisoprodol to 400 mg caffeine and placebo, ratings of calmness were increased by carisoprodol relative to caffeine (Flaten et al., 2004). Two other studies focused on psychomotor and cognitive performance: in one study, 700 mg carisoprodol did not produce impairment relative to placebo as measured by four standard neuropsychological tests (e.g., Grooved Pegboard Test) (Waterloo et al., 1997), but in another study examining a higher dose, 1050 mg, impairment occurred in four of nine tests, relative to placebo (Raffel et al., 1969).
We recently completed a drug interaction study in which the primary question of interest was whether and to what degree different skeletal muscle relaxants altered pharmacodynamic effects of oral oxycodone in healthy volunteers, and in that study two of the conditions included testing a therapeutic and supratherapeutic dose of carisoprodol alone. The drug interaction analysis, because the study drugs were administered at different times, excludes data collected for the first hour after carisoprodol was administered. The purpose of the present report is to present data from the two carisoprodol conditions as well as a placebo condition. This separate paper focusing solely on carisoprodol will include all time points after its administration in the data analysis, and thus will allow for a more complete characterization of the drug’s psychopharmacological effects. The present report, unlike the four studies involving healthy volunteers described earlier, includes subjective effects measures that are abuse liability-related (e.g., drug liking, desire to take the drug again). Also, unlike those studies, a therapeutic dose was tested in the present report, and will thus inform on how a patient prescribed this drug for musculoskeletal pain might be feeling and the degree to which that patient’s cognitive and psychomotor functioning is compromised, if at all.
2. Methods
2.1. Subjects
Requirements for participation in this IRB-approved study included: age between 21–39 years, a high school diploma or the equivalent, verbal fluency in English, and some current level of alcohol use. Exclusion criteria included: total abstention from drugs, a history of psychiatric or substance use disorders as determined from a structured interview using DSM-IV diagnostic criteria (American Psychiatric Association, 2000), or any significant medical conditions. Qualifying subjects provided written informed consent. The subject population consisted of 8 males and 7 females, with a mean age (±SD) of 27.0 (5.0) years. In the last 30 days all subjects reported drinking alcohol (average of 2.8 (1.9) drinks per week); 3 of the 15 smoked tobacco cigarettes, although none of these smoked more than 1 cigarette a day; and 5 of the 15 used marijuana (average of 1.4 (1.3) joints per week). Regarding lifetime non-medical drug use, thirteen volunteers reported use of cannabinoids (primarily marijuana), and some subjects reported use of sedatives, stimulants, opioids, and/or hallucinogens. With the exception of cannabinoids, self-reported lifetime recreational drug use of any drug from the above classes was less than 50 times in any one person, and in most cases was less than 10 times.
2.2. Drugs and doses
In the larger study there were seven experimental conditions. Two skeletal muscle relaxants, metaxalone (Skelaxin®) and carisoprodol were studied, alone and in combination with oxycodone. There were also oxycodone-alone and placebo conditions. Metaxalone had absolutely no effects in the study, and data involving it is not included in this report. Capsules were administered at three different time points at hourly intervals. During separate sessions, participants received (1) placebo; placebo; placebo, (2) placebo; 350 mg carisoprodol; placebo, (3) placebo; 700 mg carisoprodol; placebo, (4) placebo; placebo; 10 mg oxycodone, (5) placebo; 350 mg carisoprodol; 10 mg oxycodone, (6) 1600 mg metaxalone; placebo; placebo, and (7) 1600 mg metaxalone; placebo; 10 mg oxycodone. The first three conditions form the basis of this report.
Carisoprodol and placebo (lactose powder) were placed into opaque gelatin blinding capsules by an Investigational Drug Service pharmacist at the University of Chicago Hospitals.
2.3. Design and procedures
During an orientation session, participants signed a written consent form that described the study in detail. In the consent form they were told that the purpose of the study was to “see how different drugs, alone and in combination with each other, affect mood and psychomotor functioning in healthy volunteers.” They were informed that the oral drugs to be used in the study were drugs that had been approved by the Food and Drug Administration, were not experimental, and might come from one or more of the following drug classes: sedative/tranquilizer (for example, Valium®), stimulant (for example, amphetamine or speed), opiate (for example, morphine), non-prescription pain relievers (for example, Tylenol®, also known as acetaminophen, Motrin®, also known as ibuprofen, and aspirin), muscle relaxant (for example, Flexeril®), or placebo (no active drug at all).
The study was a double-blind, randomized, placebo-controlled, triple-dummy, crossover trial consisting of seven sessions (at least one week apart) that took place in a departmental laboratory from 0800-1545 hours. Subjects were instructed to not eat food the morning of sessions or use any drugs (excluding normal amounts of caffeine and nicotine) in the 24 hours prior to sessions. Upon arrival, breath alcohol, urine toxicology, and pregnancy (for females) tests were given, and subjects signed a form indicating that they had followed the food and drug restrictions.
Volunteers throughout the session were in a semirecumbent position in a hospital bed (except for bathroom breaks). At baseline, subjects completed several subjective effects forms and psychomotor tests, and their physiological status was assessed. After baseline measures were collected, subjects ingested three capsules containing metaxalone or placebo with 150 cc of water. Sixty minutes later, subjects ingested another three capsules containing carisoprodol or placebo, and 60 minutes after that ingested another three capsules containing oxycodone or placebo. At each ingestion time, subjects were told by the research technologist conducting the session that “The capsules you are about to ingest may or may not contain a drug or drugs.” Mood, psychomotor/cognitive performance, and physiological measures were assessed throughout the session at prescribed time points for 420 min after the first capsule ingestion period. After the session ended, provided they met certain discharge criteria, participants were transported to their home via a livery service.
2.4. Dependent measures
The dependent measures were assessed before the first capsule administration period (baseline), as well as at fixed time points thereafter. Data used in the analysis included only those measures that were collected starting 30 min after the second capsule ingestion period (after carisoprodol or placebo was consumed). All measures were collected at hourly intervals, and some measures were collected every 30 minutes (those will be noted below).
2.4.1. Subjective effects
Five forms were used. The first was a computerized, short form of the Addiction Research Center Inventory (ARCI), a 49-item true-false questionnaire designed to differentiate among different classes of psychoactive drugs (Haertzen, 1966; Martin et al., 1971). The ARCI yielded scores for five different scales: PCAG, sensitive to sedative effects; BG and AMP, sensitive to amphetamine-like effects; LSD, sensitive to somatic and dysphoric changes; and MBG, often described as euphoria. The second form was an adjective rating scale (ARS) derived from two questionnaires sensitive to the somatic and mood-altering effects of opioids (Fraser et al., 1961b; Preston et al., 1989). The rating scale consisted of 12 items (e.g., dry mouth, skin itchy) that the subject rated on a 5-point scale from 0 (“not at all”) to 4 (“extremely”). The third form was a locally developed visual analog scale (VAS), consisting of 28 100-mm lines, each labeled with an adjective (e.g., coasting [‘spaced out’], dreamy, lightheaded). Subjects were instructed to place a vertical mark on each line indicating how they felt at the moment, ranging from “not at all” to “extremely.” The fourth form was a locally developed Drug Effect/Drug Liking/Take Again questionnaire (DEL/TA) consisting of three items. The first item assessed the extent to which subjects currently felt a drug effect on a scale of 1 (I feel no effect from the drug(s) at all) to 5 (I feel a very strong effect). The second item assessed drug liking and disliking: subjects were asked to place a mark on a 100-mm line indicating how much they liked the drug effects as they were currently feeling them, ranging from 0 mm (dislike a lot) through 50 mm (neutral) to 100 mm (like a lot). The third item assessed how much subjects "would want to take the drug you received today again on another session, if given the opportunity” on a 100-mm line ranging from 0 mm (definitely would not) through 50 mm (don’t care) to 100 mm (definitely would). At the end of each session and 24 h later, subjects were asked to rate overall drug liking and overall wanting (to receive the drug again) on a modified version of the Drug Effect/Drug Liking/Take Again questionnaire. The VAS and DEL/TA were filled out at baseline and every 30 min thereafter up to the end of the session. The fifth form was a locally developed Post-Session Sequelae questionnaire that subjects were asked to fill out 24 h after the session; subjects were asked to rate the extent to which they felt 20 symptoms (e.g., difficulty concentrating, headache, unusual drowsiness, vomiting) since they left the laboratory, on a scale from 0 (not at all) to 4 (extremely).
2.4.2. Psychomotor performance
Five tests were used: the Digit Symbol Substitution Test (DSST), a computerized logical reasoning test (LRT), an auditory reaction time (ART) test, an eye-hand coordination (EHC) test, and a locally developed recall memory test. The DSST (Wechsler, 1958), which assesses a number of different functions, including visual scanning, mental flexibility, sustained attention, psychomotor speed, and speed of information processing (Lezak, 1995; Wetherell, 1996; Van Hoof et al., 1998), was a 1-min paper-and-pencil test that required the subject to replace digits with corresponding symbols according to a digit-symbol code listed on the top of the paper (Wechsler, 1958). The dependent measures were total number of symbols drawn and number of symbols drawn correctly. This test was done at 30-min intervals. The LRT consisted of true-false statements about the juxtaposition of the two letters A and B (e.g., A is preceded by B--true or false). The dependent measures were the total number of statements answered and number of statements answered correctly. The 1-min test assessed higher mental processes such as reasoning, logic, and verbal ability (Baddeley, 1968). A 1-min ART test measured the time it took for subjects to react to ten 50-dBA computer-generated tones which were delivered at random time intervals (Nuotto and Korttila, 1991). The mean reaction time (in seconds) to depress a computer keyboard spacebar was the dependent measure. A 1-min EHC test required the subject to track a randomly moving target (a circle) on the computer screen using a computer mouse (Nuotto and Korttila, 1991). The dependent measure was the number of times that a small plus sign, which was controlled by the mouse, deviated by more than 1 cm from the center of the target circle (i.e., termed “mistakes”). The LRT, and ART and EHC tests were administered at hourly intervals. A locally-developed memory test measured short- and long-term memory by presenting a sequential list of 15 words on the computer. These 15 words were presented in approximately 30 s. The subject was then given 120 s to write down as many words as he or she could remember. Different word lists were used for all sessions. The list was presented 150 min after the placebo or carisoprodol ingestion period. The subjects were also asked to recall the list at the end of the session (i.e., 210 min later, to test delayed free recall).
2.4.3. Physiological measures
Six physiological measures were assessed: heart rate, blood pressure, arterial oxygen saturation, respiration rate, exophoria, and pupil size.
2.5. Statistical analyses
Repeated-measures analysis of variance (ANOVA) was used for statistical treatment of the data (SigmaStat, Point Richmond, CA). The primary analysis compared peak (highest value obtained), trough (lowest value obtained), or mean effects of 0, 350, and 700 mg carisoprodol. In the peak and trough analyses, only values collected beginning 30 min after the second capsule ingestion period were included, and values were determined for each subject independent of time point. Mean effect analyses were done on those measures that were assessed only once either during or after experimental sessions. F values were considered significant for p≤0.05. When significance was achieved, the Holm-Sidak method for pairwise multiple comparison tests was done. A secondary analysis measured time course of performance on the psychomotor tests, and the subjective effects rating of “feel drug effect.”
3. Results
Table 1 summarizes mean peak or mean trough values (±SEM) of subjective and psychomotor measures in which a significant Drug effect was found.
Table 1.
Mean peak or trough scores/ratings (±SEM) of subjective and psychomotor effects measures in which a significant Drug effect was found.
| P value | placebo | carisoprodol 350 mg | carisoprodol 700 mg | |
|---|---|---|---|---|
| Subjective effects measures | ||||
| ARCI | ||||
| BGa | <0.001 | 4.4 (0.6) | 3.1 (0.5)* | 2.8 (0.5)* |
| PCAG | <0.001 | 5.9 (0.9) | 7.6 (0.8)* | 9.4 (0.8)*** |
| Adjective rating scale | ||||
| Dry mouth | 0.035 | 0.4 (0.2) | 0.4 (0.2) | 0.9 (0.3)* |
| Numb | 0.024 | 0.2 (0.1) | 0.1 (0.1) | 0.5 (0.2)** |
| VAS | ||||
| Coasting (‘spaced out’) | 0.009 | 15.3 (7.4) | 17.9 (5.6) | 34.3 (8.5)*** |
| Difficulty concentrating | 0.033 | 19.2 (7.7) | 27.7 (8.2) | 40.7 (10.7)* |
| Dizzy | 0.007 | 7.3 (5.8) | 7.7 (6.1) | 16.0 (7.0)*** |
| Dreamy | 0.002 | 18.0 (7.8) | 18.5 (6.5) | 32.9 (7.4)*** |
| Heavy or sluggish feeling | 0.018 | 20.2 (9.1) | 29.1 (8.0) | 44.0 (10.8)* |
| High (drug ‘high’) | 0.001 | 5.5 (4.0) | 10.1 (6.0) | 31.9 (9.7)*** |
| In control of bodya | 0.03 | 89.8 (5.3) | 92.9 (2.4) | 79.9 (4.9)** |
| Sleepy (drowsy, tired) | 0.021 | 40.4 (8.4) | 54.1 (8.1) | 64.9 (8.6)* |
| Drug Effect/Drug Liking/Take Again | ||||
| Feel drug | <0.001 | 2.3 (0.2) | 2.8 (0.2) | 3.6 (0.3)*** |
| Like drug | 0.037 | 58.3 (4.0) | 61.7 (3.5) | 69.9 (4.8)* |
| Take drug againa | 0.019 | 51.7 (3.4) | 43.9 (2.3) | 41.3 (4.3)* |
| Psychomotor measures | ||||
| ART (s)b | 0.045 | 0.337 (0.013) | 0.354 (0.014) | 0.358 (0.017) |
| DSST (number completed)a | <0.001 | 45.2 (2.0) | 38.9 (2.5)* | 34.5 (2.1)*** |
| DSST (number drawn correctly)a | <0.001 | 44.9 (2.1) | 38.3 (2.6)* | 33.3 (2.4)*** |
| EHC (number of mistakes)b | 0.017 | 22.5 (2.0) | 22.4 (2.1) | 26.7 (1.8)*** |
| Physiological measures | ||||
| Blood pressure: systolic (mmHg)a | 0.002 | 113.3 (3.3) | 112.2 (3.9) | 108.5 (2.7)*** |
| Heart rate (bpm) | <0.001 | 68.3 (2.1) | 73.6 (2.0)* | 76.5 (2.3)* |
p<0.05 compared with placebo
compared with carisoprodol 350 mg
p<0.05 compared with placebo and carisoprodol 350 mg
trough rating
data based on 14 subjects – one subject’s data not included because of missing data due to a file transfer error
3.1. Subjective effects
ARCI
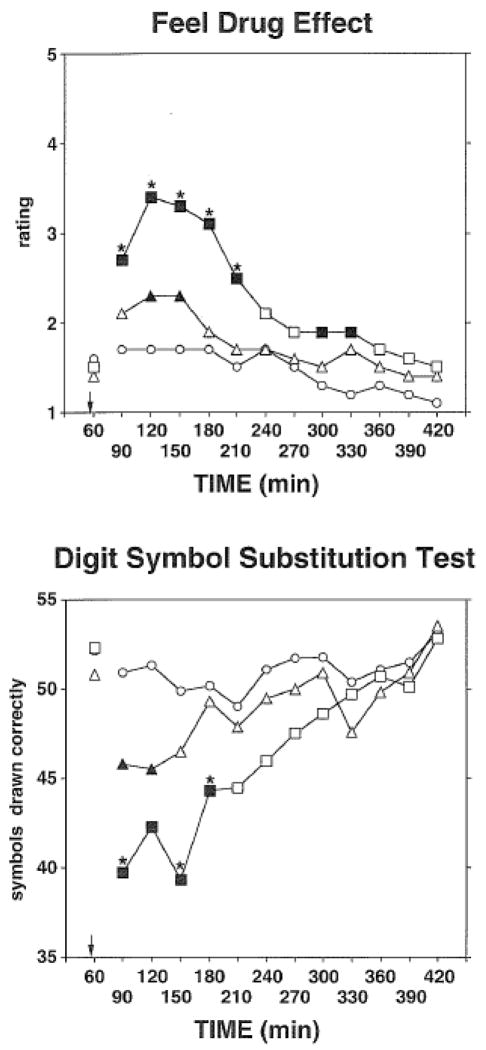
Carisoprodol at both doses decreased scores on the BG (stimulant) scale, and increased scores on the PCAG (sedative) scale, relative to placebo. Both effects were dose-related, and with PCAG scores, the higher dose differed significantly from the lower dose. ARS. Ratings of “dry mouth” were significantly higher in the 700-mg condition relative to placebo, and ratings of "numb" in the 700-mg condition were significantly higher than in the 350-mg condition. VAS. Carisoprodol produced a number of effects on this questionnaire, and the effects were confined to the large dose. In addition, the high dose differed significantly from the low dose on all of the VAS ratings in Table 1 with the exception of “difficulty concentrating,” “heavy or sluggish feeling,” and “sleepy (drowsy, tired).” DEL/TA. The top frame of Figure 1 shows mean ratings of "feel drug effect" as a function of dose and time. Both doses increased ratings relative to placebo and the effects were dose-related both in terms of magnitude of effect and also duration of effect. However, in the peak effect data analyses, only the 700 mg dose of carisoprodol significantly increased ratings of “feel drug effect” and effects were higher relative to both placebo and the 350 mg dose. Peak liking ratings were increased by 700 mg carisoprodol, but trough ratings of “take again” were also lower in this condition relative to placebo. Post-session Sequelae Questionnaire. No effects were reported in the 24 h following carisoprodol sessions, relative to the placebo session.
Figure 1.
Time course of the effects of placebo (circle), 350 mg carisoprodol (triangle) and 700 mg carisoprodol (square) on “Feel Drug Effect” ratings from the Drug Effect/Drug Liking/Take Again (DEL/TA) questionnaire (top frame) and number of symbols drawn correctly on the Digit Symbol Substitution Test (bottom frame). Each point is the mean across 15 subjects. The data which were included in the analyses included measures collected from 30 to 360 min after the administration of capsules that contained either placebo or carisoprodol – administration of the capsules occurred immediately before the 60-min time point of the session, and is indicated by the arrow in both of the graphs. Data from the 60-min time point are presented for comparison purposes (i.e., baseline), and were not included in the analyses. Solid symbols indicate a significant difference as determined by the Holm-Sidak method compared to the placebo condition for a given time point. Asterisks indicate that the 700-mg carisoprodol condition differed significantly from the 350-mg carisoprodol condition. For the Feel Drug Effect question, ratings ranged from 1–5 where 1=I feel no effect from the drug(s) at all, 2= I think I feel a mild effect, but I’m not sure, 3= I definitely feel an effect, but it is not really strong, 4= I feel a strong effect, and 5=I feel a very strong effect.
3.2. Psychomotor/cognitive performance
A significant Drug effect was obtained on the measure of ART. Although there were longer reaction times in the active drug conditions than in the placebo condition, the differences were not statistically significant. The time course analysis for ART was not significant but did show a dose-related increase one hour after the second capsule ingestion period (i.e., 0.313, 0.333, and 0.347 s in the 0, 350, and 700 mg carisoprodol conditions, respectively). On the DSST, trough measures of number of symbols drawn, and drawn correctly, were decreased by both doses of carisoprodol, and the higher dose produced more impairment than the lower dose. The bottom frame of Figure 1 shows the time course of impairment for number of symbols drawn correctly. Statistically significant impairment was noted for 1 and 2 h after administration of 350 and 700 mg of carisoprodol, respectively. Number of mistakes on the EHC test was increased by the 700 mg dose. The time course analysis was also significant, with more mistakes being made in the 700 mg dose than in the placebo condition 1 h after the second capsule ingestion period. Total number of statements answered, and total answered correctly, on the LRT in the two active drug conditions did not differ significantly from placebo in either the trough or the time course analyses. Immediate and delayed free recall was also unaffected by either dose of carisoprodol.
3.3. Physiological measures
Carisoprodol at both doses increased heart rate and the 700 mg dose decreased systolic blood pressure, relative to placebo.
4. Discussion
Carisoprodol is a musculoskeletal relaxant that is associated both with abuse and impairment (i.e., increased risk of automobile accidents) (Dougherty, 1995; Reeves et al., 1997; Venugopal et al., 2000; Bramness et al., 2004, 2007; Gorman et al., 2005; Eleid et al., 2010). In the present study acute doses of carisoprodol showed only a weak signal of abuse liability in non-drug-abusing volunteers. Although the 700 mg dose increased peak liking ratings, other abuse liability-related measures were not increased (e.g., MBG (euphoria) scale of the ARCI, VAS ratings of “having pleasant bodily sensations”), and, as well, this dose elicited reports of a reduced desire to take the drug again (compared to placebo) on another session, if given the opportunity. The relative paucity of abuse liability-related effects may not be surprising given the subject population tested – numerous studies have tested effects of other sedatives in non-drug-abusers including barbiturates and benzodiazepines, and by and large, either subjects report neutrality on hedonic scales or report disliking the drug effects (Johanson and Uhlenhuth, 1980; de Wit et al., 1984, 1989; de Wit and Griffiths, 1991). One might question the importance of testing for abuse liability-related effects of carisoprodol in this population given results from other studies showing lack of a signal of abuse potential with benzodiazepines and barbiturates, but we have found liking and other abuse liability-related effects in some non-drug abusers with other sedating drugs, including nitrous oxide, sevoflurane, propofol, and opioids (Zacny et al., 1993; Walker and Zacny 2002, 2004; Zacny and Lichtor, 2008). (In this particular study, there were no abuse liability-related effects when 10 mg of oxycodone was tested by itself.)
Carisoprodol impaired performance on some but not all tests that were used in this study. Peak ARTs in the 350- and 700-mg carisoprodol conditions were longer than in the placebo condition, although the differences were not statistically significant. Performance on the EHC was impaired by the higher dose of carisoprodol, as shown in both the peak and time course analyses. As shown in both the trough and time course analyses, performance on the DSST was impaired by both doses of carisoprodol and the impairment was dose-related. Figure 1 shows what we believe to be an interesting but potentially concerning finding - performance on the DSST in relation to the subjective rating of "feel drug effect”. When asked to rate “feel drug effect,” ratings were fairly low with the 350 mg dose (‘2’ is associated with the statement “I think I feel a mild effect, but I’m not sure”), and when DSST performance was impaired 30 min after its ingestion, subjects were reporting “feel drug effect” ratings no different from those of placebo. Therefore patients prescribed this dose might feel relatively “normal,” despite being potentially impaired (as Figure 1 would suggest), and thus be more likely to engage in some activities that might be of danger to themselves and to others (operating heavy machinery, driving a car).
Figure 1 also shows that onset of drug effects was rapid, i.e., effects were apparent 30 min after ingestion. Future studies with this drug might want to test for effects at 15-min intervals at least for the first hour after drug administration to get a more precise picture of when psychopharmacological effects of this drug first become apparent. In any event, the time at which we detected effects of carisoprodol (0.5–2 h post-ingestion) is in closer temporal contiguity to the tmax of carisoprodol (1.7 h) than meprobamate (approximately 5 h) (Bramness et al., 2005b; SOMA package insert: http://www.soma250.com/pdf/full_prescribing_info.pdf, accessed June 30, 2011). This supports the notion that the parent compound has effects in and of itself (Gonzalez et al., 2009), and that its sedating effects are not solely reliant on dealkylation to meprobamate.
As a class, musculoskeletal relaxants have received little attention by psychopharmacologists, despite their widespread use in clinical practice and their potential for misuse. We would suggest further testing of carisoprodol. Several avenues could be explored including testing even a lower dose (250 mg doses with or without aspirin are available and prescribed), and testing its interaction with other drugs including ethanol and benzodiazepines. As stated in the Introduction, the present analysis was part of a larger study in which we tested carisoprodol’s interaction with the opioid, oxycodone, and the results of that part of the study are reported elsewhere (Zacny et al., in press). Studies should also assess carisoprodol’s abuse liability in different populations that might be more likely to report positive effects, including sedative abusers (Roache and Griffiths, 1985, 1987) and moderate or heavy consumers of alcohol (de Wit et al., 1989; Evans et al., 1996). Another avenue would be to systematically examine the relationship or lack thereof between perceived degree of impairment and actual impairment. Other studies have utilized measures of metacognition and have established that subjects under the effects of benzodiazepines underestimate their performance decrements (Roache and Griffiths, 1985, 1987; Evans et al., 1990; Mintzer and Griffiths, 2003). When under the effects of alcohol or barbiturates, subjects are more accurate in estimating the degree to which they are impaired (Roache and Griffiths, 1985, 1987; Mintzer et al., 1997). The fact that at the therapeutic dose of carisoprodol participants did not feel strong drug effects, yet were definitely impaired (as measured by the DSST), might indicate that they would respond in a similar manner to that of people under the effects of benzodiazepines in estimating their performance. Finally, we would suggest that intra-study comparisons of the subjective and psychomotor effects of carisoprodol to other muscle relaxants such as cyclobenzaprine (Flexeril) and methocarbamol (Robaxin) would be important from a clinical standpoint. Such studies would inform physicians which muscle relaxants have safer profiles. Our experience with metaxalone, however, in which no effects were noted, would suggest that a subchronic dosing regimen might be needed since these drugs differ in their pharmacokinetic profiles, and to simulate the clinical milieu in which these drugs are usually taken more than once a day.
In conclusion, carisoprodol at a therapeutic dose had few subjective effects, but did impair performance on the DSST, a test that taps into complex neuropsychological processes (Lezak, 1995; Wetherell, 1996; Van Hoof et al., 1998). A higher dose produced more robust subjective effects and psychomotor impairment. These findings, in addition to evidence in the scientific literature that the drug is abused and is associated with impairment of a serious nature (traffic accidents), would argue that further study is warranted to more systematically characterize the psychopharmacological effects of carisoprodol.
Highlights.
In non-drug-abusing volunteers, carisoprodol, at a supratherapeutic dose of 700 mg, had several subjective effects, but at a therapeutic dose of 350 mg only had minimal subjective effects.
In contrast, both doses of carisoprodol impaired psychomotor performance, including a test that tapped into both cognitive and motor functioning.
Patients prescribed the therapeutic dose might feel relatively “normal” and thus be more likely to engage in some activities that might be of danger to themselves and to others (operating heavy machinery, driving a car).
Acknowledgments
Research was supported in part by grant RO1 DA23969 from the National Institute on Drug Abuse. We thank Karin Kirulis for screening potential subjects and conducting the structured interviews, Jenny M. Jun and Nicole Olson for assistance in conducting the experimental sessions, and Sandra Gutierrez for assistance in data analysis. The authors have no conflicts of interest to report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders DSM-IV-TR (Text Revision) Washington DC: American Psychiatric Association; 2000. [Google Scholar]
- Baddeley AD. A three-minute reasoning test based on grammatical transformation. Psychonom Sci. 1968;10:341–2. [Google Scholar]
- Bramness JG, Skurtveit S. Carisoprodol should be taken off the market. South Med J. 2008b;101:1074–5. doi: 10.1097/SMJ.0b013e318184ac60. [DOI] [PubMed] [Google Scholar]
- Bramness JG, Buajordet I, Skurtveit S. The role of pharmacoepidemiological studies in the market withdrawal of carisoprodol (Somadril) in Europe. 2008a;18:167–72. [Google Scholar]
- Bramness JG, Skurtveit S, Morland J. Impairment due to intake of carisoprodol. Drug Alcohol Depend. 2004;74:311–8. doi: 10.1016/j.drugalcdep.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Bramness JG, Skurtveit S, Morland J, Engeland A. The risk of traffic accidents after prescriptions of carisoprodol. Accident Analysis Prevention. 2007;39:1050–5. doi: 10.1016/j.aap.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Bramness JG, Morland J, Sordid HK, Rudberg N, Jacobsen D. Carisoprodol intoxications and serotonergic features. Clin Toxicol. 2005a;1:39–45. doi: 10.1081/clt-45020. [DOI] [PubMed] [Google Scholar]
- Bramness JG, Skurtveit S, Gullicksen M, Breilid H, Steen VM, Morland J. The CYP2C19 genotype and the use of oral contraceptives influence the pharmacokinetics of carisoprodol in healthy human subjects. Eur J Clin Pharmacol. 2005b;61:499–506. doi: 10.1007/s00228-005-0970-1. [DOI] [PubMed] [Google Scholar]
- Dalen P, Alvan G, Wakelkamp M, Olsen H. Formation of meprobamate is catalyzed by CPYP2C19. Pharmacogenetics. 1996;6:387–94. doi: 10.1097/00008571-199610000-00002. [DOI] [PubMed] [Google Scholar]
- de Wit H, Griffiths RR. Testing the abuse liability of anxiolytic and hypnotic drugs in humans. Drug Alcohol Depend. 1991;28:83–111. doi: 10.1016/0376-8716(91)90054-3. [DOI] [PubMed] [Google Scholar]
- de Wit H, Pierri J, Johanson CE. Reinforcing and subjective effects of diazepam in nondrug-abusing volunteers. Pharmacol Biochem Behav. 1989;33:205–13. doi: 10.1016/0091-3057(89)90451-6. [DOI] [PubMed] [Google Scholar]
- de Wit H, Pieri J, Johanson CE. Assessing pentobarbital preference in normal volunteers using a cumulative dosing procedure. Psychopharmacology. 1989;99:416–21. doi: 10.1007/BF00445569. [DOI] [PubMed] [Google Scholar]
- de Wit H, Uhlenhuth EH, Johanson CE. Lack of preference for flurazepam in normal volunteers. Pharmacol Biochem Behav. 1984;21:865–9. doi: 10.1016/s0091-3057(84)80066-0. [DOI] [PubMed] [Google Scholar]
- Dillon C, Paulose-Ram R, Jirsch R, Gu Q. Skeletal muscle relaxant use in the United States. Data from the Third National Health and Nutrition Examination Survey (NHANES III) Spine. 2004;29:892–6. doi: 10.1097/00007632-200404150-00014. [DOI] [PubMed] [Google Scholar]
- Dougherty RJ. Carisoprodol should be a controlled substance. Arch Fam Med. 1995;4:582. doi: 10.1001/archfami.4.7.582. [DOI] [PubMed] [Google Scholar]
- Douglas JF, Ludwig BJ, Schlosser A. The metabolic fate of carisoprodol in the dog. J Pharmacol Exp Ther. 1962;138:21–7. [Google Scholar]
- Eleid MF, Krahn LE, Agrwal N, Goodman BP. Carisoprodol withdrawal after internet purchase. Neurologist. 2010;16:262–4. doi: 10.1097/NRL.0b013e3181aa917e. [DOI] [PubMed] [Google Scholar]
- Evans S, Funderburk F, Griffiths RR. Zolpidem and triazolam in humans: behavioral and subjective effects and abuse liability. J Pharmacol Exp Ther. 1990;255:1246–55. [PubMed] [Google Scholar]
- Evans SM, Griffiths RR, de Wit H. Preference for diazepam, but not buspirone, in moderate drinkers. Psychopharmacology (Berl) 1996;123:154–63. doi: 10.1007/BF02246172. [DOI] [PubMed] [Google Scholar]
- Fass JA. Carisoprodol legal status and patterns of abuse. Annal Pharmacother. 2010;44:1962–7. doi: 10.1345/aph.1P339. [DOI] [PubMed] [Google Scholar]
- Flaten MA, Simonsen T, Med C, Olsen H. Drug-related information generates placebo and nocebo responses that modify the drug response. Psychosom Med. 1999;61:250–5. doi: 10.1097/00006842-199903000-00018. [DOI] [PubMed] [Google Scholar]
- Flaten MA, Simonsen T, Zahlsen K, Aamo T, Sager G, Olsen H. Stimulant and relaxant drugs combined with stimulant and relaxant information: a study of active placebo. Psychopharmacology. 2004;176:426–34. doi: 10.1007/s00213-004-1886-7. [DOI] [PubMed] [Google Scholar]
- Fraser HF, Essig CF, Wolbach AB. Evaluation of carisoprodol and phenyamidol for addictiveness. Bull Narcotics. 1961a;13:3–7. [Google Scholar]
- Fraser HF, van Horn GD, Martin WR, Wolbach AB, Isbell H. Methods for evaluating addiction liability. (a) “attitude” of opiate addicts toward opiate-like drugs, (b) a short-term “direct” addiction test. J Pharmacol Exp Ther. 1961b;133:371–87. [PubMed] [Google Scholar]
- Gonzalez LA, Gatch MB, Taylor CM, Bell-Horner CL, Forster MJ, Dillon GH. Carisoprodol-mediated modulation of GABAA receptors: in vitro and in vivo studies. J Pharmacol Exp Ther. 2009;329:827–37. doi: 10.1124/jpet.109.151142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman JM, Rohatgi G, Rismiller DJ. Treatment of carisoprodol dependence: a case report. J Psychiat Prac. 2005;11:347–52. doi: 10.1097/00131746-200509000-00008. [DOI] [PubMed] [Google Scholar]
- Haertzen CA. Development of scales based on patterns of drug effects, using the Addiction Research Center Inventory (ARCI) Psychol Rep. 1966;18:163–94. doi: 10.2466/pr0.1966.18.1.163. [DOI] [PubMed] [Google Scholar]
- Hoiseth G, Karinen R, Sorlid HK, Bramness JG. The effect of scheduling and withdrawal of carisoprodol on prevalence of intoxications with the drug. Basic Clin Pharmacol Toxicol. 2009;105:345–9. doi: 10.1111/j.1742-7843.2009.00459.x. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: diazepam. Psychopharmacology. 1980;71:269–73. doi: 10.1007/BF00433061. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological Assessment. 3. New York: Oxford University Press; 1995. [Google Scholar]
- Logan BK, Case GA, Gordon AM. Carisoprodol, meprobamate, and driving impairment. J Forensic Sci. 2000;45:619–23. [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–58. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Lorazepam and scopolamine: a single-dose comparison of effects on human memory and attentional processes. Exp Clin Psychopharmacol. 2003;11:56–72. doi: 10.1037//1064-1297.11.1.56. [DOI] [PubMed] [Google Scholar]
- Mintzer MZ, Guarino J, Kirk T, Roache JD, Griffiths RR. Ethanol and pentobarbital: comparison of behavioral and subjective effects in sedative drug abusers. Exp Clin Psychopharmacology. 1997;5:203–15. doi: 10.1037//1064-1297.5.3.203. [DOI] [PubMed] [Google Scholar]
- Nuotto EJ, Korttila K. Evaluation of a new computerized psychomotor test battery: effects of alcohol. Pharmacol Toxicol. 1991;68:360–5. doi: 10.1111/j.1600-0773.1991.tb01253.x. [DOI] [PubMed] [Google Scholar]
- Olsen H, Koppang E, Alvan G, Morland J. Carisoprodol elimination in humans. Ther Drug Monit. 1994;16:337–40. doi: 10.1097/00007691-199408000-00001. [DOI] [PubMed] [Google Scholar]
- Preston KL, Bigelow GE, Bickel WK, Liebson IA. Drug discrimination in human postaddicts: agonist-antagonist opioids. J Pharmacol Exp Ther. 1989;250:184–96. [PubMed] [Google Scholar]
- Raffel SC, Swink R, Lampton TD. The influence of chlorphenesin carbamate and carisoprodol on psychological test scores. Current Ther Res. 1969;11:553–60. [PubMed] [Google Scholar]
- Reeves RR, Burke RS. Is it time for carisoprodol to become a controlled substance at the federal level? South Med J. 2008;101:127–8. doi: 10.1097/SMJ.0b013e3181612062. [DOI] [PubMed] [Google Scholar]
- Reeves RR, Burke RS. Carisoprodol: abuse potential and withdrawal syndrome. Curr Drug Abuse Rev. 2010;3:33–8. doi: 10.2174/1874473711003010033. [DOI] [PubMed] [Google Scholar]
- Reeves RR, Carter OS, Pinkofsky HB. Use of carisoprodol by substance abusers to modify the effects of illicit drugs. South Med J. 1999;92:441. doi: 10.1097/00007611-199904000-00032. [DOI] [PubMed] [Google Scholar]
- Reeves RR, Pinkofsky HB, Carter OS. Carisoprodol: a drug of continuing abuse. J Am Osteopath Assoc. 1997;97:723–4. doi: 10.7556/jaoa.1997.97.12.723. [DOI] [PubMed] [Google Scholar]
- Roache JD, Griffiths RR. Comparison of triazolam and pentobarbital: performance impairment, subjective effects and abuse liability. J Pharmacol Exp Ther. 1985;234:120–33. [PubMed] [Google Scholar]
- Roache JD, Griffiths RR. Lorazepam and meprobamate: behavioral effects and abuse liability. J Pharmacol Exp Ther. 1987;243:978–88. [PubMed] [Google Scholar]
- Robertson MD, Marinetti LJ. Carisoprodol – effects on human performance and behavior. Forensic Sci Rev. 2003;15:1–10. [PubMed] [Google Scholar]
- Roth RA, Vinson DR, Kim S. Carisoprodol-induced myoclonic encephalopathy. J Toxicol Clin Toxicol. 1998;36:609–12. doi: 10.3109/15563659809028058. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. [accessed May 2, 2011];Results from the 2009 National Survey on Drug Use and Health: Detailed Tables, Illicit Drug Use Tables. 2010a Available at: http://oas.samhsa.gov/NSDUH/2k9NSDUH/tabs/Sect1peTabs47to92.htm#Tab1.90A.
- Substance Abuse and Mental Health Services Administration. Analytic Group: Nonmedical Use of Pharmaceuticals Visits. Rockville, MD: Center for Behavioral Health Statistics and Quality; 2010b. [accessed May 2, 2011]. Drug Abuse Warning Network, 2009: Selected Tables of National Estimates of Drug-Related Emergency Department Visits. Available at: https://dawninfo.samhsa.gov/data/report.asp?f=Nation/NMUP/Nation_2009_NMUP_ED_Visits_by_Drug. [Google Scholar]
- Toth PP, Urtis H. Commonly used muscle relaxant therapies for acute low back pain; a review of carisoprodol, cyclobenzaprine hydrochloride, and metaxalone. Clin Ther. 2004;26:1355–67. doi: 10.1016/j.clinthera.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Van Hoof JM, Jogems-Kosterman BM, Sabbe BC, Zitman FG, Hulstijn W. Differentiation of cognitive and motor slowing in the Digit Symbol Test (DST): differences between depression and schizophrenia. J Psychiatr Res. 1998;1998(32):99–103. doi: 10.1016/S0022-3956(98)00057-0. [DOI] [PubMed] [Google Scholar]
- Venugopal D, Deepak G, Murali N, Kumar KB, Sharma PS. A case report of carisoprodol dependence. Indian J Psychiatry. 2000;42:211–3. [PMC free article] [PubMed] [Google Scholar]
- Walker DJ, Zacny JP. Analysis of the reinforcing and subjective effects of different doses of nitrous oxide using a free-choice procedure. Drug Alcohol Depend. 2002;66:93–103. doi: 10.1016/s0376-8716(01)00188-0. [DOI] [PubMed] [Google Scholar]
- Walker DJ, Beckman NJ, Zacny JP. Reinforcing and subjective effects of the volatile anesthetic, sevoflurane. Drug Alcohol Depend. 2004;76:191–201. doi: 10.1016/j.drugalcdep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Wetherell A. Performance tests. Environ Health Perspect. 1996;104 (Suppl 2):247–73. doi: 10.1289/ehp.96104s2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The measurement and appraisal of adult intelligence. Vol. 1958. Baltimore: Williams and Wilkins; 1958. [Google Scholar]
- Waterloo K, Flaten MA, Simonsen T, Olsen H. The effect of carisoprodol on performance on repeatedly administered neuropsychological tests. Human Psychopharmacol. 1997;12:393–6. [Google Scholar]
- Zacny JP, Lichtor SA. Within-subject comparison of the psychopharmacological profiles of oral oxycodone and oral morphine in non-drug-abusing volunteers. Psychopharmacology (Berl) 2008;196:105–16. doi: 10.1007/s00213-007-0937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, Paice JA, Coalson DW. Subjective and psychomotor effects of carisoprodol in combination with oxycodone in healthy volunteers. Drug Alcohol Depend. 2011 doi: 10.1016/j.drugalcdep.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny JP, Lichtor JL, Thompson W, Apfelbaum JL. Propofol at a subanesthetic dose may have abuse potential in healthy volunteers. Anesth Analg. 1993;77:544–52. doi: 10.1213/00000539-199309000-00020. [DOI] [PubMed] [Google Scholar]