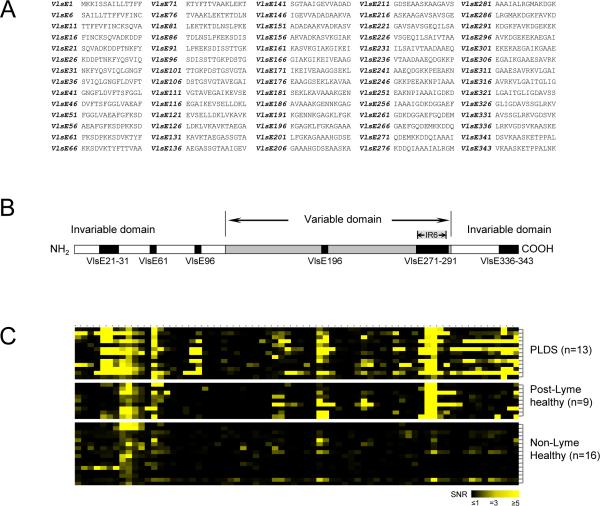
Figure 1. Epitope mapping of VlsE.
A) Synthesized peptides of the VlsE protein of B. burgdorferi B31 used for preparation of microarrays. Peptide notation is according to the amino acid number (in the VlsE protein sequence) of the first residue of each peptide. B) Diagrammatic structure of VlsE showing the 14 peptides (representing 6 contiguous regions) that were found to be the main epitopes of the protein targeted by antibodies in individuals with a history of Lyme disease. These peptides had significantly higher level of IgG antibody reactivity towards them in the post-Lyme groups (n=13 for PLDS, n=9 for post-Lyme healthy) in comparison to the non-Lyme healthy control group (n=16) (p<0.05). C) Heat map of antibody reactivity for tested specimens towards the 70 synthesized VlsE peptides (VlsE1 through VlsE343 from left to right, corresponding to panel B).