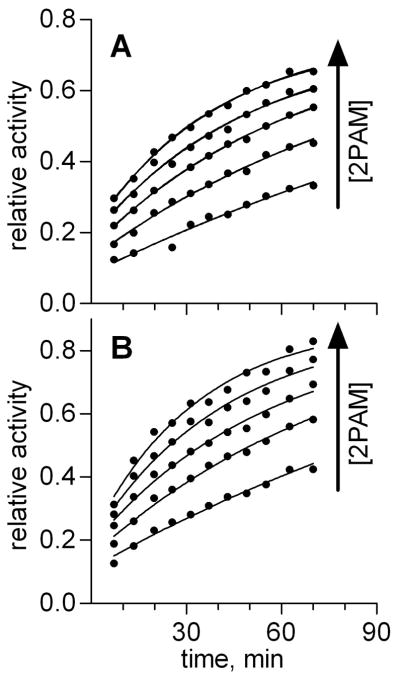
Fig. 1. 2-PAM oxime-mediated reactivation of SpGB-W541H6Δ (A) and SpGB- FL (B) BChE enzymes.
Enzyme was inhibited with SpGBc, then reactivated with various concentrations of 2-PAM, as described in Materials and Methods. At various time points during the reactivation period, aliquots of enzyme were assessed for their ability to hydrolyze BTC, and the resulting rates of hydrolysis were plotted as a function of the amount of time enzyme incubated with 2-PAM. For clarity, the rates of hydrolysis were normalized to non-inhibited control samples. Data points for the five 2-PAM concentrations were simultaneously fit to Eq. 3 to determine the bimolecular reactivation rate constant (kr) for each enzyme.