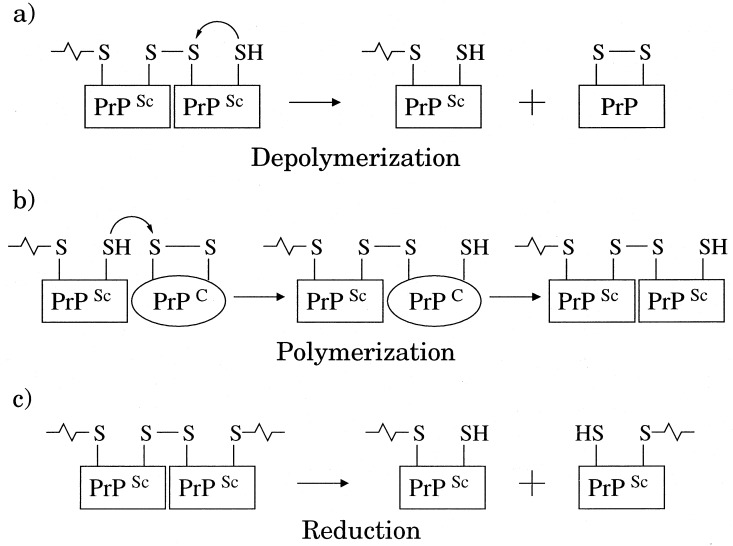
Figure 1.
(a) Depolymerization reaction in which a terminal thiolate of a disulfide-bonded polymer attacks the preceding intermolecular disulfide bond, producing a shortened polymer and a monomer with an intact intramolecular disulfide bond. (b) Polymerization reaction in which a terminal thiolate of the disulfide-bonded polymer attacks the intramolecular disulfide bond of the cellular form PrPC, lengthening the polymer. Presumably, the PrPC molecule is destabilized by its association to the scrapie-form polymer, and hence, its disulfide bond is not as well protected as in free solution. (c) Reduction of an intermolecular disulfide bond. Experiments have demonstrated that the rates of reshuffling and reduction depend strongly on the stability of the tertiary structure protecting the disulfide bond (18–20).