Figure 2.
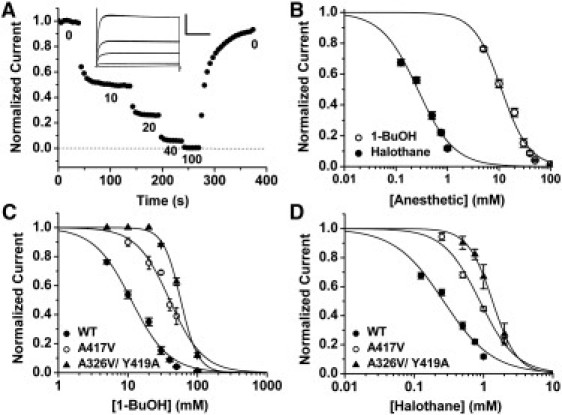
Dose-inhibition analysis of the wild-type K-Shaw2 channel and representative Ala/Val mutants. (A) Normalized current versus time plot of wild-type K-Shaw2. Upon application of increasingly higher doses of 1-BuOH (mM), there is an incremental inhibition of the current. This inhibition reaches equilibrium and is reversible upon washout. The inset shows the whole-oocyte K-Shaw2 currents corresponding to the experiment shown in the main panel evoked by a step from −100 to +60 mV; scale for inset indicates 4 μA (y axis) and 100 ms (x axis). All mutants were subjected to this experimental strategy. (B) Semilogarithmic plots of normalized current versus anesthetic concentration for wild-type K-Shaw2. The solid lines depict the best Hill equation fits, which yielded the following parameters for halothane: K0.5 = 0.26 mM, nH = 1.2; and the following best-fit parameters for 1-BuOH: K0.5 = 11.2 mM, nH = 1.6. (C) The effects of Ala/Val mutations on the 1-BuOH dose-inhibition relation. A417V shifted the relation to the right (K0.5 = 39.4 mM, nH = 1.7) and the double mutation A326V/Y419A caused an even larger shift and increased the apparent cooperativity (K0.5 = 57.7 mM, nH = 3.4). (D) The effects of Ala/Val mutations on the halothane dose-inhibition relation. A417V showed a rightward shift (K0.5 = 0.88, nH = 1.7), and the double mutation A326V/Y419A exhibited an even larger shift (K0.5= 1.34, nH = 2.5). In all cases (B–D), the points represent the average of 4–45 independent determinations.
