Figure 7.
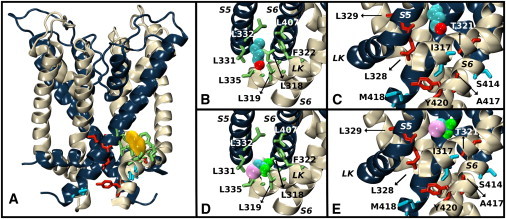
Average equilibrium conformation of the K-Shaw2 closed-state structure bound to 1-BuOH and halothane. (A) Side view of K-Shaw2 with backbones colored in navy blue and off-white. The density blob (yellow) depicts 1-BuOH bound to site 3. Residue side chains < 4.7 Å from 1-BuOH are represented as green sticks (Table 1). (B) Close-up view of 1-BuOH in site 3. (C) Close-up view of 1-BuOH effector sites. Red-colored side chains indicate that Ala/Val mutations had an unfavorable energetic impact on the inhibition of K-Shaw2 by 1-BuOH, and blue-colored side chains indicate a favorable energetic impact. (D) As in panel B, but displaying halothane in site 3. (E) As in panel C, but displaying the effects of mutations on the inhibition by halothane.
