Figure 3.
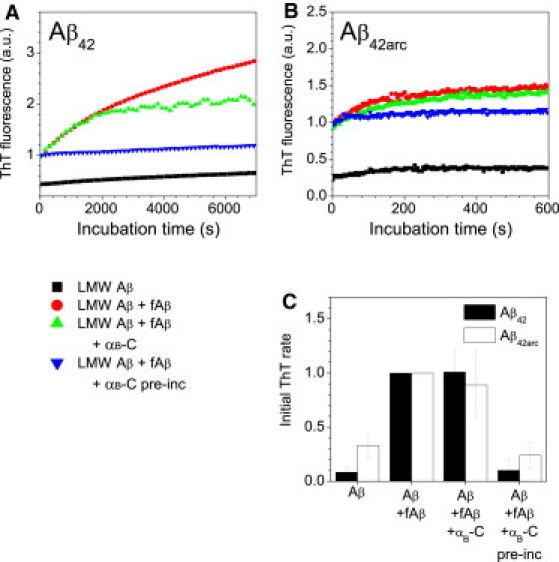
Inhibition of Aβ fibril elongation by αB-crystallin binding. Aβ fibril elongation kinetics for Aβ42 (A) and Aβ42arc (B) fibrils (fAβ) at room temperature in buffer A in the presence and absence of αB-crystallin. The solutions examined were LMW Aβ alone (black squares), LMW Aβ with fibril seed (red circles), LMW Aβ with fibril seed and a low concentration of αB-crystallin (green triangles), and LMW Aβ with αB-crystallin bound to fibril seeds (blue triangles). The fluorescence traces plotted are the average of three replicates and are reported relative to the starting fluorescence of solutions containing Aβ fibrils but no αB-crystallin. Control solutions of LMW Aβ with αB-crystallin displayed behavior similar to that observed for LMW Aβ alone. Solutions containing αB-crystallin and ThT alone showed no change in fluorescence over these timescales. (C) The initial elongation rates for Aβ42 (black bars) and Aβ42arc (white bars) were determined from the gradient of the linear fit to the first portion of data (1000 s for Aβ42, 200 s for Aβ42arc) for three repeats. Initial rates have been normalized with respect to Aβ elongation in the presence of Aβ seeds and the absence of αB-crystallin. Reported rates are the mean and standard deviation of the three repeats. The fibril seeds were preincubated with αB-crystallin for 1 h at room temperature. Concentrations of Aβ fibrils and αB-crystallin (when present) were ∼0.8 μM and 0.4 μM, respectively. Concentrations of LMW Aβ were 9.7 μM and 1.8 μM for Aβ42 and Aβ42arc, respectively.
