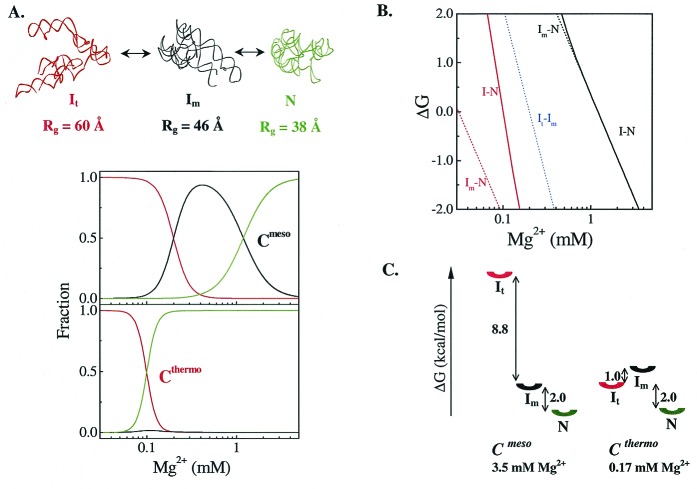
Figure 1.
Equilibrium folding models for a thermophilic (Cthermo) and a mesophilic (Cmeso) RNA. (A) The Mg2+-dependent equilibrium folding for both Cthermo and Cmeso has two identical intermediates, It and Im. Fractional population of each species is shown. (B) The free energy changes for each transition. The solid lines represent the experimentally determined ΔG as a function of Mg2+ concentration as defined by ΔG = −RT ln([N]/([It] + [Im])). (C) Free energy diagrams at Mg2+ concentrations where both ribozymes have the same functional stability, ΔGI-to-N = −2.0 kcal/mol, corresponding to 0.16 and 3.5 mM Mg2+ for the thermophilic and the mesophilic ribozyme, respectively. Near the folding transition, the It state is less stable than the Im state, so that the latter largely defines the functional stability of the native mesophilic ribozyme. The relative stability of the intermediates is switched so that the It is the defining species for the stability of the thermophilic ribozyme. For Cmeso, an increase in the stability of Im would shift it and the native species down by the same amount, and not alter the functional stability.