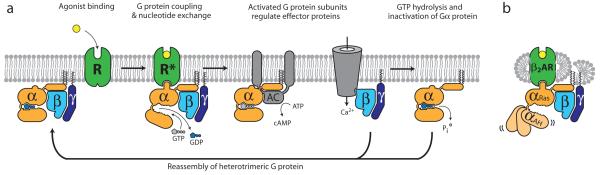
Figure 1. G protein cycle for the β2AR-Gs complex.
a, Extracellular agonist binding to the β2AR leads to conformational rearrangements of the cytoplasmic ends of transmembrane segments that enable the Gs heterotrimer (α, β, and γ) to bind the receptor. GDP is released from the α subunit upon formation of β2AR-Gs complex. The GTP binds to the nucleotide-free α subunit resulting in dissociation of the α and βγ subunits from the receptor. The subunits regulate their respective effector proteins adenylyl cyclase (AC) and Ca2+ channels. The Gs heterotrimer reassembles from α and βγ subunits following hydrolysis of GTP to GDP in the α subunit. b, The purified nucleotide-free β2AR-Gs protein complex maintained in detergent micelles. The Gαs subunit consists of two domains, the Ras domain (αRas) and the α-helical domain (αAH). Both are involved in nucleotide binding. In the nucleotide-free state, the αAH domain has a variable position relative the αRas domain.