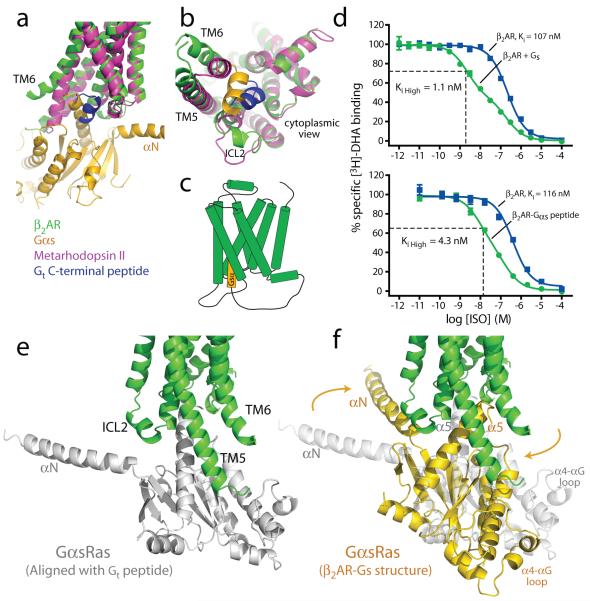
Figure 6. Possible sequence of β2AR-Gs complex formation.
a, b, Comparison of the β2AR-Gs structure (green and gold) with metarhodopsin II31 (PDB ID: 3PQR) (purple) bound with the carboxyl-terminal peptide of transducin (blue). TM7 has been omitted in panel a to better visualize the G proteins. c, Cartoon of the β2AR-Gαs peptide fusion construct used in the binding experiments (d). d, Competition binding experiments between [3H]-DHA and full agonist isoproterenol. Top panel shows binding data (reproduced from Rasmussen et al., 201112) on β2AR reconstituted in HDL particles with and without Gs heterotrimer. The fraction of β2AR in the Ki high state for the β2AR with Gs is 0.55. Bottom panel shows binding to β2AR and a β2AR-Gαs peptide fusion expressed in Sf9 cell membranes. The fraction of β2AR in the Ki high state for the β2AR-Gαs peptide fusion is 0.68. e, The initial interaction of agonist bound β2AR and GαsRas may involve an orientation of the carboxyl-terminus of GαsRas similar to that of the carboxyl-terminal peptide of transducin in the structure of metarhodopsin II. f, The final position of GαsRas on the β2AR as observed in the β2AR-Gs complex.