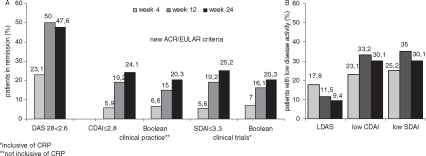
Figure 2.
(A)Patients achieving remission according to the different assessment tools DAS28 ((0.56×TJC2) + (0.28×SJC2) + (0.7×ln (ESR)) + (0.014 × PtGA)<2.6) and the new ACR/EULAR criteria for clinical practice (Boolean-criteria SJC, TJC, PtGA all ≤1) and CDAI (SJC + TJC + PhGA + PtGA) ≤2.8) and clinical trials (Boolean-criteria SJC, TJC, PtGA, CRP all ≤1 and SDAI (SJC+ TJC + PhGA + PtGA + CRP (mg/dl)) ≤3.3 ). (B) Patients achieving low disease activity (DAS >2.6 and ≤3.2; CDAI >2.8 and ≤10; SDAI >3.3 and ≤11). (All data shown ‘as observed’). CRP, C-reactive protein; CDAI, Clinical Disease Activity Index; DAS28, 28-joint count Disease Activity Score; ESR, erythrocyte sedimentation rate; PhGA, physician global assessment; PtGA, patient global assessment; SDAI, Simplified Disease Activity Index; SJC, swollen joint count; TJC, tender joint count.