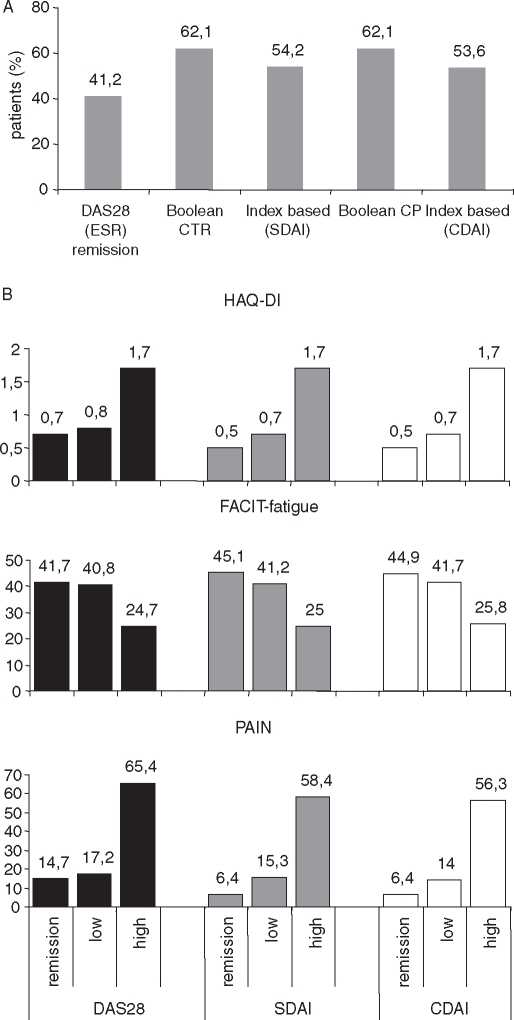
Figure 3.
(A)Proportion of patients with HAQ-DI remission (<0.5) according to the five remission criteria (erythrocyte sedimentation rate (ESR); clinical trial (CTR); clinical practice (CP)). (B) Impact of clinical remission according to DAS28, CDAI or SDAI on patient-related outcome HAQ-DI (scaled from 0 to 3, 0=no disability), FACIT-fatigue (higher scores indicate better quality of life) and pain (visual analogue scale 0=no pain, 100=worst pain) after 24 weeks. The more stringent criteria CDAI and SDAI tended to have a better patient-related outcome on the HAQ-DI and pain, but not in the FACIT fatigue score. Noteworthy also patients with low disease activity benefited clearly from tocilizumab treatment. CDAI, Clinical Disease Activity Index; DAS28, 28-joint count Disease Activity Score; FACIT, Functional Assessment of Chronic Illness; HAQ-DI, Health Assessment Questionnaire-Disability Index; SDAI, Simplified Disease Activity Index.