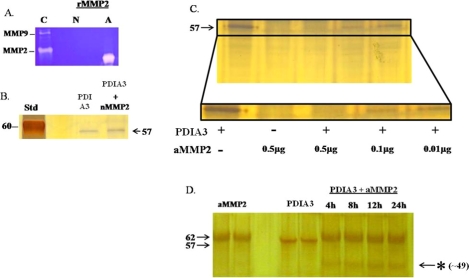
FIG. 3.
Validation of protein disulfide isomerase A3 as a target of MMP2 action. A) Gel zymography demonstrating gelatinolytic activity of an active recombinant MMP2 (A) and a nonactive MMP2 (N). Conditioned media from Ovcar-3 cells was used as a positive control (C) for MMP2 and MMP9 activity. B) Protein gel of purified PDIA3 (0.1 μg) and PDIA3 incubated with nMMP2 (0.9 μg) for 24 h. The nMMP2 was unable to cleave PDIA3. The molecular weight of monomeric PDIA3 is 57 kDa. C) Protein gel of purified PDIA3 (0.1 μg) incubated with increasing concentrations of aMMP2 (0.01, 0.1, 0.5 μg) for 24 h. The single band of PDIA3 was diminished after incubation with aMMP2. Similar results were obtained in five different experiments. D) Protein gradient gel (4%–20%) of active MMP2 (aMMP2, 0.5 μg), purified PDIA3 (0.1 μg), and PDIA3 incubated with aMMP2 (0.5 μg) for varying amounts of time. The asterisk (*) indicates a breakdown product of PDIA3 at approximately 49 kDa.