Figure 5.
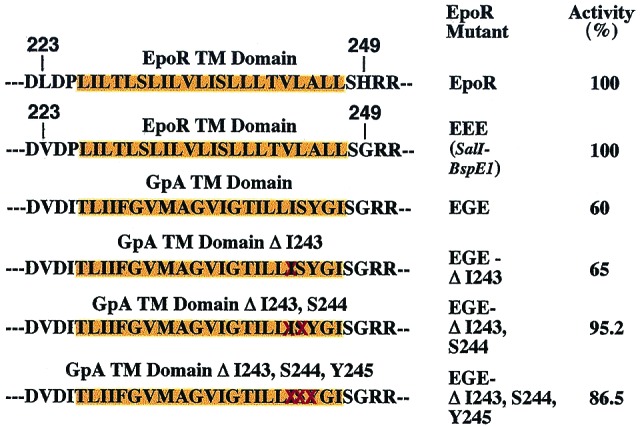
EpoR can tolerate replacement of its TM with that of GpA. TM domains (depicted over orange background) and flanking amino acid residues of the EGE chimeric molecules are shown. DNA oligonucleotides coding for the GpA TM domain or the indicated deletion mutants (deleted residues are crossed in red) were cloned in the EpoR EEE construct, which contains the two point mutations Leu223 → Val and His249 → Gly flanking the TM domain. Activity is defined by percent of proliferation of Ba/F3 cells expressing equal numbers of wild-type or mutant EpoRs, as revealed by similar GFP fluorescence, measured 4 days after plating in medium supplemented with Epo at 1 unit/ml. A representative experiment in which samples were counted in duplicate is shown. No activity was detected in the absence of Epo.
