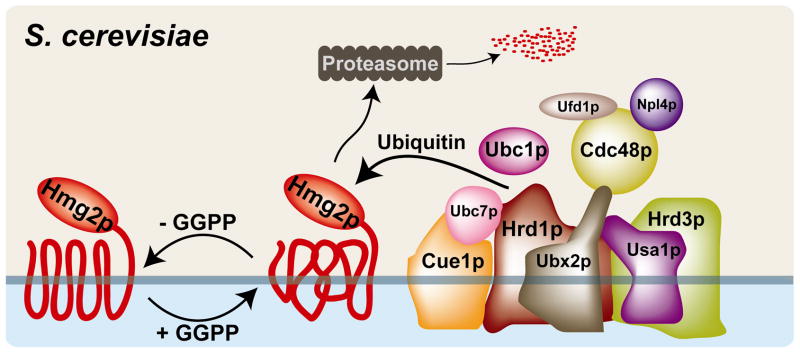
Figure 5. Hmg2p levels are controlled post-translationally by geranylgeranyl pyrophosphate and the HRD complex in S. cerevisiae.
Geranylgeranyl pyrophosphate alters the conformation of the Hmg2p N-terminal membrane domain, promoting recognition and ubiquitination by the membane-bound, multi-subunit HMG-CoA reductase degradation (HRD) complex. Hrd1p, a membrane-spanning ubiquitin E3 ligase with homology to the mammalian E3 ligase gp78, utilizes two E2 ubiquitin conjugating enzymes, Ubc7p and Ubc1p. Ubc7p, a soluble protein that interacts with the membrane through the integral membrane protein Cue1p, is the primary E2 for Hrd1. Hrd3 is involved in substrate recognition and delivery to the HRD complex. Usa1p plays a role in Hrd1p function and Hrd1p self-regulation. Ubx2p is an integral membrane protein that recruits the Cdc48p/Npl4p/Ufd1p complex. Cdc48p, a hexameric ATPase and homolog of mammalian p97/VCP, acts in retrotranslocation of both luminal and membrane-bound HRD substrates. The HRD complex contains other components, including Der1p, Kar2p and Yos9p, that are not required for Hmg2p degradation and are not shown.