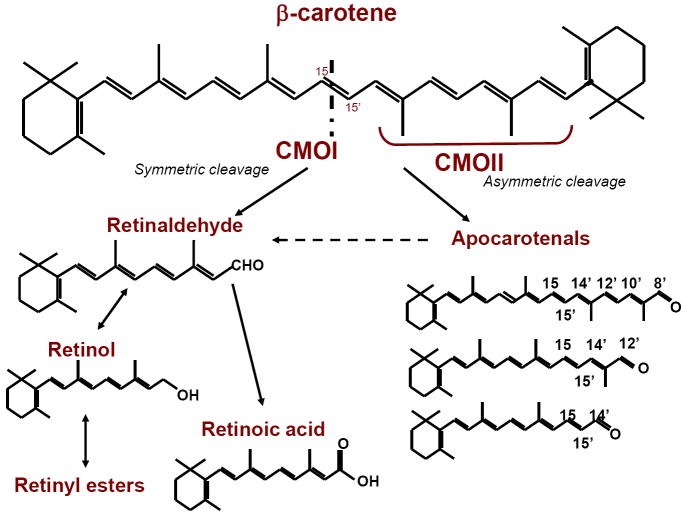
Figure 3. Cleavage of β-carotene and its conversion into retinoids.
When β-carotene is cleaved symmetrically by the action of the 15,15’-oxygenase enzyme (CMOI) two molecules of retinaldehyde are produced. Retinaldehyde can then be oxidized to form retinoic acid or can be reduced to retinol, which is further converted into retinyl ester, the storage form of vitamin A in tissues. In vivo, 95% of retinoids arising from β-carotene are produced by this pathway. However, β-carotene can also be cleaved asymmetrically by the action of 9’,10’-oxygenase enzyme (CMOII) producing apocarotenals, which can be converted to retinaldehyde by chain shortening. Through this pathway, one molecule of β-carotene yields one molecule of retinaldehyde. Interestingly, Ziouzienkova and colleagues [153] showed that apocarotenals may also function as ligands for PPAR and RXR receptors, inhibiting vitamin A signaling.