Abstract
Ultrashort echo time (UTE) imaging and sweep imaging with Fourier transformation (SWIFT) are powerful techniques developed for imaging ultrashort T2 species. However, it can be challenging to implement them on standard clinical MRI systems due to demanding hardware requirements. In this paper the limits of what is possible in terms of the minimum echo time (TE) and repetition time (TR) with 3D radial gradient-echo (GRE) sequences, which can be readily implemented on a standard clinical scanner, are investigated. In addition, a new 3D radial GRE sequence is introduced, called CODE (COncurrent Dephasing and Excitation). The unique feature of CODE is that the initial dephasing of the readout gradient is performed during RF excitation, which allows CODE to effectively achieve echo times on the order of ~0.2 ms and larger in a clinical setting. The minimum TE achievable with CODE is analytically described and compared with a standard 3D radial GRE sequence. CODE was implemented on a clinical 3T scanner (Siemens 3T MAGNETOM Trio), and both phantom and in vivo human knee images are shown for demonstration.
Keywords: UTE imaging, 3D radial gradient-echo imaging, CODE, radial sampling, short TE, short TR
INTRODUCTION
Recently, multiple subspecialty areas of MRI research have experienced a surge of interest in techniques to image spins with very short transverse relaxation times, T2 and T2*. For biomedical MRI, the musculoskeletal system in particular contains a majority of short T2 components in connective tissues such as cortical and trabecular bone, meniscal and labral tissues, ligaments and tendons. Such tissues are not easily detected with conventional MRI sequences. Hence, methods like ultrashort echo-time (UTE) imaging (1,2) and sweep imaging with Fourier transformation (SWIFT) (3,4) have been developed to image tissue water signals with T2 components as short as 100 – 200 μs. With gradient-recalled echo (GRE) imaging, it is generally assumed that the shortest achievable echo time (TE) is ~2 ms when imaging with a clinical scanner. With UTE and SWIFT on the other hand, the delay (or dead time) between excitation and the start of signal acquisition is made very short by acquiring a free induction decay (FID) instead of a gradient-recalled echo. One point worth mentioning here is that the common use of TE to describe the delay between excitation and FID acquisition is inconsistently defined. We therefore prefer to use the alternative terminology of “acquisition delay” in the description of UTE and SWIFT.
In both UTE and SWIFT, k-space is filled in a radial manner (i.e., with “spokes” radiating out from k = 0). In UTE imaging, after radiofrequency (RF) excitation, data acquisition begins as quickly as possible while the readout gradient is being ramped on, to minimize signal loss and blurring (5). To capture signals from extremely short T2 spins, UTE is typically implemented with a short RF pulse (which usually necessitates high peak RF amplitude, B1max) and high gradient slew rate. The shortest achievable acquisition delay is ~ 20 – 100 μs on most clinical scanners, although ~ 8 μs is the shortest acquisition delay that has been reported (6). UTE also encounters challenges relating to non-uniform sampling as a result of sampling data on the gradient ramp. Gridding of non-uniformly acquired data in k-space is particularly difficult since the gradient ramp is not perfectly linear in many cases and the gradients on different axes can have different temporal responses (7). In SWIFT, the gradient field is constant while acquiring the data in a time-shared manner with the RF excitation. That is, the gradient amplitude is at a constant value while signal is acquired in gaps inserted in a broadband frequency-swept excitation pulse. The acquisition delay is limited only by the time needed for the coil ring down (and up) and to switch between transmit and receive modes. This time can be as short as 2 μs when using the latest electronic components in transmit/receive switches (in single transmit/receive coil configuration) or in pin-diode switches (in separate transmit/receive coil applications).
As a modified version of UTE imaging, water- and fat-suppressed projection MR imaging (WASPI) was also proposed in which the gradient is ramped on prior to the RF pulse and held constant until FID sampling is complete (8). In WASPI and related techniques (9,10), unlike UTE, data is acquired not on the gradient ramp, but on the constant gradient, avoiding the challenging problem of non-uniform sampling. However, since WASPI employs a non-selective square RF pulse in the presence of the gradient, the pulse length has to be very short to minimize the signal variation across the object, due to the sinc-shaped excitation profile of the square pulse. As a result, the requirement for high peak RF (B1max) can be more of a limitation for these techniques than for UTE and SWIFT. In addition, due to the FID sampling with a very fast rate (typically, 5 - 25 μs per point), data points close to the origin of k-space are not measured during the dead time of the receiver, and thus, an additional short data collection is sometimes needed to recover the lost points (11-13).
Both UTE and SWIFT rely on achieving superior hardware performance, and as a result, are currently undergoing intense development and testing in many different laboratories. For these reasons, standardized versions of these sequences are not yet widely available on commercial MRI scanners. Many applications, including musculoskeletal studies of tendons, ligaments, and meniscus, require detection of spins with T2 values only in the 1 – 10 ms range, rather than in the tens to hundreds of microsecond range. In such cases, a GRE sequence using the shortest possible TE may suffice.
The purpose of the present study was therefore to investigate the limits of what is possible in terms of minimum TE and repetition time (TR) with 3D radial GRE imaging using a standard clinical scanner. In addition, a new 3D radial GRE sequence is introduced, called CODE (COncurrent Dephasing and Excitation). As can be recognized from its name, the unique feature of CODE is that the initial dephasing of the readout gradient is performed during RF excitation, which allows CODE to effectively achieve TE ≥ ~0.2 ms on a clinical scanner. In this article, the minimum TE and TR achievable with CODE are analytically described and compared with the more standard 3D radial GRE sequence (rGRE). It is also shown that CODE can be implemented on a clinical 3T scanner, and both phantom and in vivo human knee images are shown for demonstration.
METHODS
Pulse sequence and data acquisition
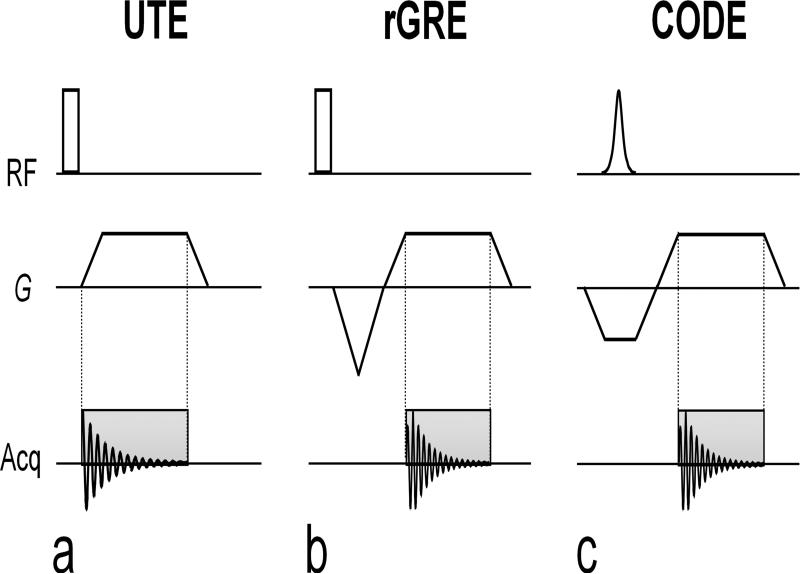
Simplified schematic diagrams of 3D UTE, rGRE, and CODE sequences are shown in Fig. 1. In UTE (Fig. 1a) and rGRE (Fig. 1b), a non-selective square pulse is used for spin excitation, whereas in CODE (Fig. 1c), a shaped pulse is needed to excite a relatively uniform flip angle in the presence of a slab-select gradient (Gss). The essential feature of the UTE technique is that the FID acquisition begins while ramping up the readout gradient (Gro) to achieve the shortest possible acquisition delay, without applying an initial dephasing (or pre-dephasing) gradient. Conversely, in rGRE and CODE, immediately following a pre-dephasing gradient which has the opposite polarity of Gro, a gradient echo is acquired after the readout gradient has reached a plateau. The unique feature of CODE, however, is that slab selection and pre-dephasing are performed with the same gradient. In other words, a pre-dephasing gradient for the frequency-encoded readout is applied during, not after, spin excitation in CODE. Following excitation, acquisition is delayed only by the need to ramp the gradient in one direction, which allows the minimum TE to be less than that of rGRE. In CODE, a frequency-selective RF pulse such as a sinc or a hyperbolic secant pulse (sech/tanh) can be used for the spin excitation in the presence of the slab-select gradient, provided that the thickness of the selected slab is wider than the largest dimension of the object being imaged.
FIG. 1.
Pulse sequence diagrams of 3D UTE, 3D radial GRE (rGRE), and CODE. (a) In the 3D UTE, a square pulse is used for spin excitation and data acquisition starts during the readout gradient ramp to achieve the shortest acquisition delay possible. Since no dephasing gradient is applied for readout, a FID is acquired during acquisition. (b) In rGRE, a square pulse is also used for excitation. Data is acquired during the readout gradient following an initial dephasing (or pre-dephasing) gradient, and thus, a gradient echo is acquired instead of the FID. A partial gradient-echo acquisition scheme is employed to minimize TE. (c) In CODE, slab selection and pre-dephasing are performed with the same gradient. In other words, a pre-dephasing gradient is applied during, not after, spin excitation. Hence, a gradient echo is acquired as in rGRE. A slab-selective pulse such as a sinc or a hyperbolic secant pulse can be used for spin excitation. Because the acquisition time will usually be much longer than the pulse duration (i.e. Tacq is on the order of a few milliseconds or less, whereas Tp is usually ~100 – 300 microseconds), the peak of the gradient echo forms soon after the start of the acquisition period and, thus, the gradient echoes used to fill k-space in each radial direction are asymmetric about the origin of k-space.
In rGRE and CODE, a partial gradient echo acquisition is exploited to minimize TE. The gradient ramps in the x, y, and z axes are required to have the same ramp time (τ) so that the gradients in these axes can start at the same time despite their different amplitudes and thus different rise times. The achievable minimum τ depends on the performance of a gradient system and, in CODE, is equal to the sum of the minimum τ of Gss and Gro (Fig. 1c). Here the minimum τ of Gss and Gro is given by dividing Gss and Gro by the maximum slew rate (SR), respectively. The radial sampling is typically performed with an isotropic angular spacing to cover a sphere in k-space, where the end of the spoke follows a spiral path on the surface of the sphere referred to as view orders (14). Several view-order spirals can be interleaved to increase the total number of projections.
When imaging short T2 spins with CODE, the pulse length (Tp) is kept as short as possible to achieve the shortest TE within permissible B1max and specific absorption rate (SAR) constraints. In the usual implementation of CODE for human studies, Tp is typically ~100 - 300 microseconds, while the acquisition time (Tacq) is much longer, on the order of a few milliseconds or less, depending on the resolution and acquisition bandwidth. In this Tp << Tacq case, the peak of the gradient echo forms soon after the start of the acquisition period. As a result, the gradient echoes used to fill k-space in each radial direction (i.e., the spokes) are asymmetric about the origin of k-space. Due to the asymmetric feature of the gradient-echoes, the highest spatial resolution achieved with rGRE and CODE is given by FOV / {2(nacq – nshort) – 1}, where FOV is the field-of-view, nacq is the number of sampled k-space points in data acquisition, nshort is the number of sampled points on the short side of the asymmetric echo.
In the current implementation of rGRE and CODE, images are reconstructed offline using gridding and fast Fourier transformation (FFT). Gridding is performed to distribute the radially sampled data onto Cartesian coordinates by convolving with a Kaiser-Bessel function for interpolation. Then 3D FFT is performed, followed by deapodization which removes the interpolation effect caused by the gridding. For density correction in the gridding process, a two-step iterative gridding/regridding of ones is performed as proposed by Pipe et al. (15). To assign each measurement value to its correct k-space location, each channel was phased separately. Using the phase of the average projection, a single linear function was fitted in a weighted least-squares manner, where the weight was the magnitude of the average projection. From the slope of the linear function, the correct timing of k-space was determined.
The minimum TE achievable with CODE
In this section, the conditions for achieving the shortest TE with CODE are analyzed. For convenience, the slab thickness (THK) is assumed to be the same as the FOV. Under this assumption, the pulse bandwidth (BW) and the acquisition bandwidth (SW) are linearly proportional only to the magnitude of Gss and Gro, respectively. In this case, three different situations are considered for the analysis of the shortest TE achievable with CODE, which are: BW > SW (or Gss > Gro), BW = SW (or Gss = Gro), and BW < SW (or Gss < Gro). These are referred to as case 1, 2, and 3, respectively.
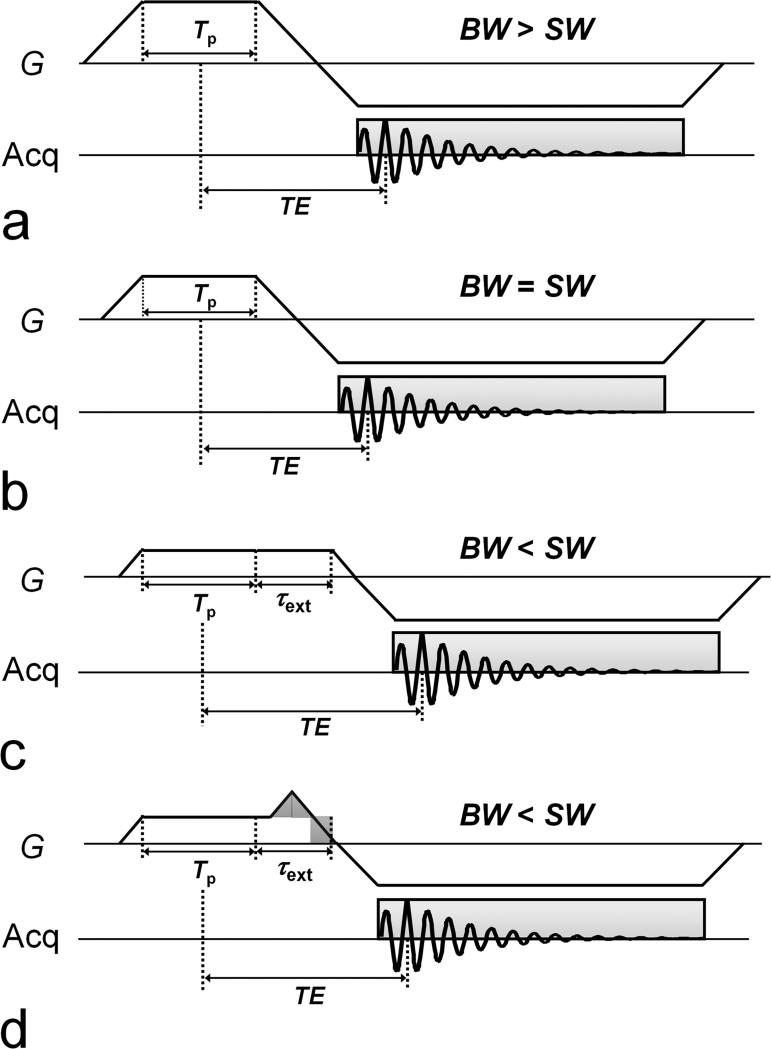
To begin, let us compare case 1 (Fig. 2a) with case 2 (Fig. 2b) in terms of achievable minimum TE. With TE defined as the time between the center of the RF pulse and the peak of the echo, in cases 1 and 2,
| [1] |
where Tshort is the duration of the short-side of the asymmetric echo, τss is the ramp time needed for Gss to reach a plateau (= Gss/SR), and τro is the ramp time needed for Gro to reach a plateau (= Gro/SR). Provided that Tp, SW, and FOV are the same in the two cases, TE1 > TE2 because τss,1 > τss,2 and Tshort,1 > Tshort,2, where subscripts 1 and 2 indicate the case. Here, τss,1 > τss,2 because BW1 > SW = BW2, and thus, Gss,1 > Gss,2. Tshort,1 > Tshort,2 can be inferred from the fact that the gradient area with respect to time for pre-dephasing in case 1 is greater than that in case 2, i.e., Gss,1(Tp/2 + τss,1) > Gss,2(Tp/2 + τss,2). Therefore, it is concluded that case 2 always offers a shorter TE than case 1.
FIG. 2.
Pulse sequence diagrams of CODE for three different situations, i.e., BW > SW (or Gss > Gro), BW = SW (or Gss = Gro), and BW < SW (or Gss < Gro), which are referred to as case 1 (a), case 2 (b), and case 3, respectively. Particularly in case 3, the gradient area for the pre-dephasing is less than that for the readout, and as a result additional time for dephasing with Gss is needed to keep the echo peak from occurring during the readout gradient ramp. Two sequence schemes are possible for this purpose. In the case of the basic scheme (c), which is referred to as case 3a, Gss is simply extended having the same constant value as the original setup. However, in case 3b (d), a part of the extended gradient is replaced with gradient ramps (i.e., ramp-up and ramp-down) with an area (shaded triangle) equal to that of a part of the extended gradient to be replaced (shaded rectangle). The latter always provides a shorter TE than the former by the amount of time corresponding to the width of the shaded rectangle. As proven herein (see text), case 2 provides the shortest TE among the possible schemes, except in some specific circumstances (e.g., small FOV), case 3b provides the shortest TE.
In case 3, the gradient area for the pre-dephasing is less than that for the readout, and as a result additional time for dephasing with Gss is needed to keep the echo peak from occurring during the readout gradient ramp. As shown in Figs. 2c and 2d, two sequence schemes are possible for this purpose. In the case of the basic scheme (Fig. 2c), which will be referred to as case 3a, Gss is simply extended in time using the same (constant) Gss value. However, in case 3b (Fig. 2d), a part of the extended gradient is replaced with gradient ramps (i.e., ramp-up and ramp-down) with an area (shaded triangle) equal to that of the part of the extended gradient to be replaced (shaded rectangle). Case 3b always provides a shorter TE than case 3a by the amount of time corresponding to the width of the shaded rectangle.
Now let us compare case 2 with case 3a. While TE2 is given by Eq. [1], TE3a is given by adding the extended dephasing time (τext) to Eq. [1]. Provided that Tp, SW, and FOV (= THK) are the same in the two cases,
| [2] |
| [3] |
where Tp,2 = Tp,3 = Tp and τro,2 = τro,3 = τro. In Eq. [3], when it is also assumed that Tshort,2 = Tshort,3, τext can be expressed in terms of Tp and τss,2 by considering that the gradient area (A) for the predephasing in case 3a has to be equal to that in case 2, i.e., A2 = A3. For convenience, τss,3a can be assumed to be ατss,2, where 0 < α < 1, because BW3 > BW2 and thus, Gss,3 > Gss,2. In this case, the expression for τext in terms of Tp and τss,2 is obtained from the equation A2 = A3, which is,
| [4] |
When using Eqs. [2], [3], and [4], the numerical difference between TE3a and TE2 is also given in terms of α, Tp, and τss,2,
| [5] |
Since both first and second terms in Eq. [5] are always positive due to the assumption that 0 < α < 1, TE3a is always greater than TE2, and thus, it is concluded that case 2 always provides a shorter TE than case 3a.
Next, let us compare case 2 with case 3b in which a part of the extended Gss is replaced with gradient ramps. As shown in Fig. 2d, TE3b is shorter than TE3a by τss,3a. Thus, TE3b is obtained by simply subtracting τss,3a from Eq. [3],
| [6] |
In this case, the numerical difference between TE3b and TE2 is given by
| [7] |
using Eqs. [2], [4], and [6]. Unlike ΔTE3a in Eq. [5], ΔTE3b is not always positive, which means that TE3b is shorter than TE2 in some cases. When determining the condition for ΔTE3b > 0, the constraint needs to be considered that τext should be no less than 2τss,3a in order for the gradient ramp not to begin during the slab-selective excitation, i.e., τext ≥ 2τss,3a (=ατss,2). Because the analysis for ΔTE3b is complicated and tedious, let us go directly to the conclusion without presenting the detailed description of it here: For α larger than 0.5 (i.e., 0.5 < α < 1), case 2 always provides a shorter TE than case 3b. For α smaller than 0.5 (i.e., 0 < α < 0.5), case 2 provides a shorter TE than case 3b when Tp > 0.5τss,2, which occurs in human applications using a large THK. In the event that Tp < 0.5τss,2, which can be the case in animal studies (e.g., THK ≤ 5.2 cm when Tp = 0.1 ms, BW = 10 kHz, and SR = 170 mT/m/ms), case 3b provides a shorter TE than case 2 for αi < α < 0.5, where αi is the point at which ΔTE3b = 0. In summary, case 2 provides the shortest TE achievable with CODE, except for case 3b when Tp < 0.5τss,2 for αi < α < 0.5.
In Table 1, the minimum TE values and maximum flip angles achieved with CODE on a 3T Siemens Trio scanner are provided using a knee coil and a body coil. A sinc pulse was used with R = 10, where R is defined as the product of Tp and BW (i.e., R = Tp·BW). nshort was set to be 4. The Siemens phantom D170 with distilled water (including 8.2g NaC2H2O2 + 9.6g C3H3O3Li per 1000g H2O) was used for coil loading. The minimum TE values were measured for two different FOVs (25 cm and 40 cm), using different Tp values ranging from 0.1 - 0.25 ms in 0.05 ms increments. With Tp = 0.1 ms, the achievable minimum TE was 0.22 ms and 0.18 ms for FOV = 25 cm and 40 cm, respectively, and these were 0.31 ms and 0.27 ms with Tp = 0.25 ms. The maximum flip angles were also measured using the two coils. When using the knee coil, the maximum flip angle was 6° and 12° with Tp = 0.1 ms and 0.25 ms, respectively, and these were 3° and 9° for the body coil. As seen here, when a very short TE is needed, large flip angles cannot be attained with CODE due to the peak B1 or SAR limitation.
Table 1.
The minimum TE and maximum flip angles achieved with CODE on a 3T MAGNETOM Trio scanner, using a sinc pulse with R = 10.
| FOV (cm) | Pulse length (ms) | Min TE (ms) | Max flip angle (degree) | |
|---|---|---|---|---|
| Knee coil | 25 | 0.10 | 0.22 | 6 |
| 0.15 | 0.26 | 9 | ||
| 0.20 | 0.29 | 11 | ||
| 0.25 | 0.31 | 12 | ||
| 40 | 0.10 | 0.18 | 6 | |
| 0.15 | 0.22 | 9 | ||
| 0.20 | 0.25 | 11 | ||
| 0.25 | 0.27 | 12 | ||
| Body coil | 25 | 0.10 | 0.22 | 3 |
| 0.15 | 0.26 | 5 | ||
| 0.20 | 0.29 | 6 | ||
| 0.25 | 0.31 | 9 | ||
| 40 | 0.10 | 0.18 | 3 | |
| 0.15 | 0.22 | 5 | ||
| 0.20 | 0.25 | 6 | ||
| 0.25 | 0.27 | 9 |
*MAGNETOM Trio scanner, using a sinc pulse with R = 10.
The minimum TE achievable with rGRE
In the rGRE sequence (Fig. 1b), spins are excited by a short square pulse which is not slab selective (Gss = 0), followed by a pre-dephasing gradient (Gdeph), and then by Gro. In order to minimize TE, the pre-dephasing gradient can be replaced by gradient ramps only (i.e., ramp up and ramp down) without a gradient plateau. In this case, the minimum TE is given by
| [8] |
where τdeph is the ramp time for the pre-dephasing gradient to reach a setup value (τdeph = Gdeph/SR). In the following section, the equations above will be analyzed in detail to determine which sequence, rGRE or CODE, affords the shortest TE values.
Comparison of the minimum TE between CODE and rGRE
Here, it is assumed that peak B1 or SAR determines the minimum Tp. Hence, in the following comparison of CODE and rGRE, B1max or SAR is set equal in the two sequences and set to produce the same flip angle. Under these conditions, the duration of the square pulse used in rGRE will be shorter than that of the frequency-selective pulse (e.g., a sinc) used in CODE. Thus, it is reasonable to assume that the pulse lengths in CODE and rGRE are Tp and cTp, respectively, where c is the ratio of the pulse length in these two sequences, whereby 0 < c ≤ 1. All the readout parameters such as SW, FOV, τro, and Tshort are also assumed to have the same values in both CODE and rGRE.
For the comparison of the minimum TE achievable with CODE (Eq. [1]) and rGRE (Eq. [8]), it is convenient to describe τdeph in Eq. [8] in terms of τro and Tshort, considering that the gradient area of the dephasing (Adeph) is equal to that of the rephasing (Areph). Given τdeph = Gdeph/SR,
| [9] |
On the other hand, Areph is given by the sum of the area of the gradient ramp and the readout gradient corresponding to Tshort, which is,
| [10] |
Given Adeph = Areph (i.e., Eq. [9] = Eq. [10]), τdeph can be expressed as
| [11] |
Using Eq. [11] and recalling that the pulse length in rGRE was assumed to be cTp, the minimum TE achievable with rGRE (Eq. [8]) is rewritten as
| [12] |
As proven in the previous section, the minimum TE in CODE in most human applications is accomplished with case 2, i.e., when BW = SW. In this case, τss = τro, and thus, the minimum TE in CODE (Eq. [1]) can also be rewritten as
| [13] |
From Eqs. [12] and [13], the condition can be derived for which TErGRE = TECODE. A quadratic equation in terms of τro is obtained by using the fact that Eq. [12] = Eq. [13], which is,
| [14] |
Since Tshort = Tp/2 in CODE (i.e., in case 2), the coefficient of the second term in Eq. [14] is also expressed in terms of c and Tp. Hence, Eq. [14] is rewritten as
| [15] |
Eq. [15] has two real solutions and its non-trivial solution is
| [16] |
Since τro = Gro/SR = SW/(γFOV·SR), the condition for TECODE < TErGRE is
| [17] |
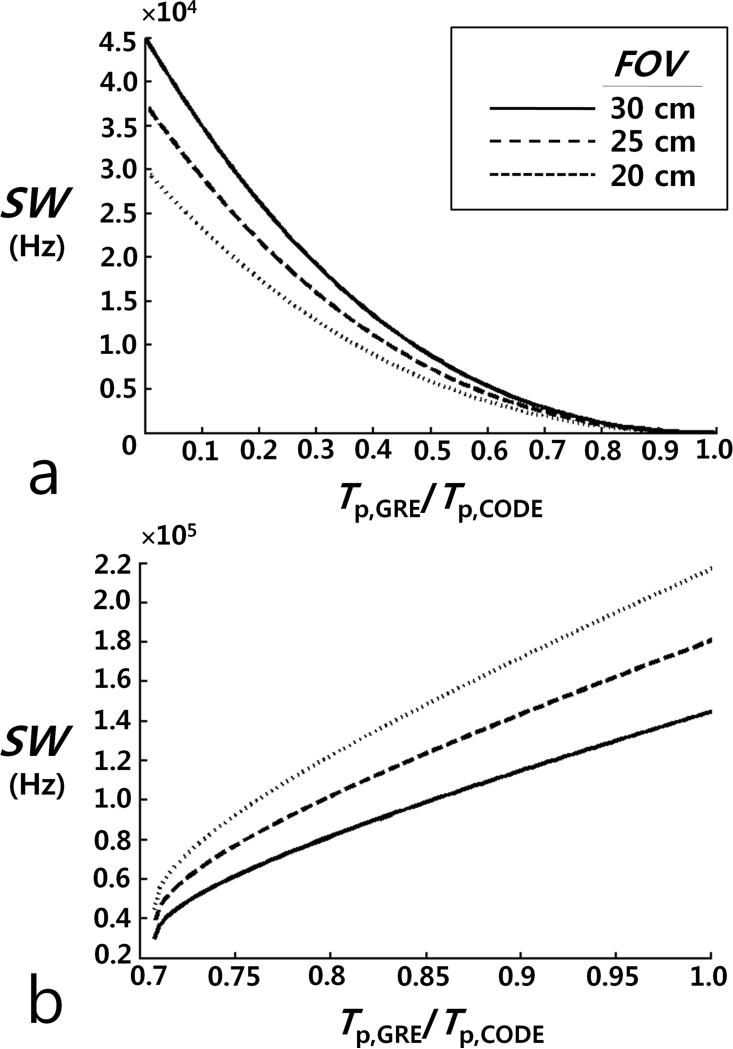
where γ is the gyromagnetic ratio. In Fig. 3a, SW is plotted as a function of c (= Tp,rGRE/Tp,CODE) for different FOV values (= 20, 25, and 30 cm), as c varies between 0 and 1. CODE achieves a shorter TE than rGRE, provided that SW is larger than the plotted value for a given c; that is, when Eq. [17] is satisfied.
FIG. 3.
Plots showing the relationship between SW and the ratio of the pulse length (Tp,GRE) in rGRE to the pulse length (Tp,CODE) in CODE for different FOVs = 20, 25, and 30 cm, (a) when the achievable minimum TE of CODE is equal to that of rGRE and (b) when the achievable minimum TR of CODE is equal to that of rGRE. (a) For a given Tp,GRE/Tp,CODE, CODE provides a shorter TE than rGRE if SW is larger than the plotted value. (b) While CODE has a longer TR than rGRE for Tp,GRE/Tp,CODE < 0.7071, it has a shorter TR than rGRE for Tp,GRE/Tp,CODE ≥ 0.7071, provided that SW is smaller than the plotted value.
Table 2 provides examples of the minimum TE and TR achievable with CODE and rGRE for two different FOVs. For the calculation, a square pulse was used for rGRE and a standard sinc pulse with 3 lobes (R = 10) was used for CODE. The slew rate was set equal to that achievable with a 3T Siemens MAGNETOM Trio system (gradient slew rate = 170 mT/m/ms). A spoiling gradient was not considered for the calculation of TR. A maximum value of 500 Hz was assumed for γB1/2π, which is a readily attainable value with a whole-body RF coil. The flip angle was set to 3.9° in both pulse sequences. For both CODE and rGRE, BW = SW = 80 kHz, nacq = 128, and nshort = 4. In CODE, the duration of the sinc pulse (Tp,sinc) was set to be 100 μs. With the same B1max value used with the sinc pulse, the duration of the square pulse (Tp,square) is 21.67 μs. In the case of equal SAR produced by sinc and square pulses, Tp,square = 62.07 μs. The minimum TE and TR values that could be achieved with CODE were 0.188 ms and 1.877 ms, respectively, for FOV = 25 cm, and they were 0.155 ms and 1.811 ms for FOV = 40 cm. In the case of the same B1max, the minimum TE and TR achievable with rGRE were 0.217 ms and 1.821 ms, respectively, for FOV = 25 cm, and 0.172 ms and 1.759 ms for FOV = 40 cm. In the case of the same SAR, these were 0.238 ms and 1.863 ms, respectively, for FOV = 25 cm, and 0.193 ms and 1.801 ms for FOV = 40 cm. In both cases, as compared with rGRE, CODE has a slightly shorter minimum TE and slightly longer minimum TR. The minimum TE and TR decreases as FOV increases due to the reduction of τss, τro, and τdephs.
Table 2.
The minimum TE and TR (in ms) theoretically achievable with CODE and rGRE.
| FOV | CODE | rGRE | ||
|---|---|---|---|---|
| same B1max | same SAR | |||
| 25 cm | TEmin | 0.188 | 0.217 | 0.238 |
| TRmin | 1.847 | 1.821 | 1.863 | |
| 40 cm | TEmin | 0.155 | 0.172 | 0.193 |
| TRmin | 1.811 | 1.759 | 1.801 | |
*Assumes slew rate of 3T Siemens MAGNETOM Trio scanner (170 mT/m/ms)
EXPERIMENTS
CODE and rGRE sequences were implemented and tested on a clinical 3T MAGNETOM Trio scanner (Siemens, Erlangen, Germany), with a 40 mT/m maximum peak gradient amplitude and a 170 mT/m/ms maximum slew rate. The rGRE and CODE sequence codes were written in-house. In all cases of CODE imaging, a tailored sinc pulse available in the Siemens pulse-shape library was used. This tailored sinc pulse delivers a very flat excitation profile in the pulse bandwidth. A 12-element head coil (Siemens “Head Matrix”) for signal reception in combination with a transmit body coil was used for imaging the resolution phantom. In vivo human knee images were acquired with a transmit/receive birdcage knee coil (Siemens “CP Extremity”). Imaging of normal human volunteers was performed according to the procedure approved by the Institutional Review Board of the University of Minnesota Medical School. For offline image reconstruction, gridding was performed onto two-times oversampled Cartesian grid using a Kaiser-Bessel interpolation kernel with a diameter of 4. No additional corrections were applied to compensate for gradient timing errors and non-linearity, which, however, does not imply that CODE is robust to gradient delays.
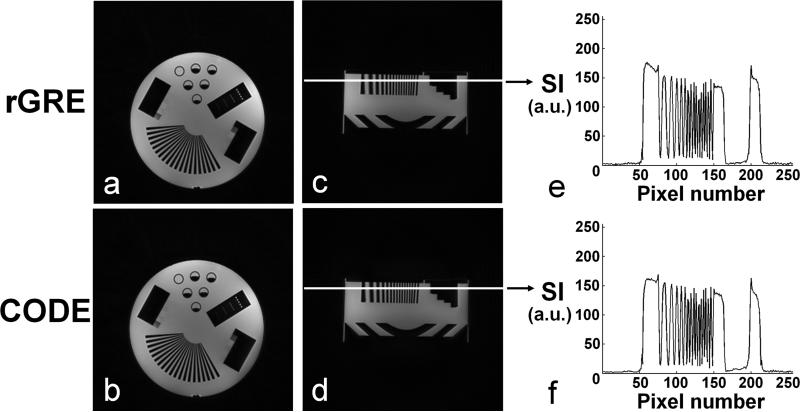
Imaging of a resolution phantom (Nuclear Associates 76-903, Fluke Biomedical) was performed to assess the relative image quality and performance of the two sequences. In Fig. 4, phantom images are shown in axial (a and b) and sagittal (c and d) planes. Signal intensity (SI) profiles are also shown along the white solid line in the sagittal images obtained with rGRE (e) and CODE (f), respectively. In all cases, flip angle = 5°, TR = 3.4 ms, FOV (= THK) = 30 cm, and number of projections = 128 k, to achieve 1.0 mm3 isotropic resolution in a scan time of ~6 min. rGRE was performed with TE = 0.22 ms and Tp,square = 0.05 ms. CODE was performed with TE = 0.19 ms and a sinc pulse of duration 0.1 ms (= Tp,sinc) and R = 6.4.
FIG. 4.
Resolution phantom images acquired using rGRE and CODE. Phantom images are shown in axial (a and b) and sagittal (c and d) planes. Signal intensity (SI) profiles are also shown along the white solid line in the sagittal images obtained with rGRE (e) and CODE (f), respectively. In all cases, flip angle = 5°, TR = 3.4 ms, and FOV (= slab width) = 30 cm. The number of projections = 128 k. Scan time = ~6 min. Isotropic resolution = 1.0 mm3. For 3D radial GRE images (a and c), a square pulse was used with Tp,square = 0.05 ms and TE = 0.22 ms. For CODE images (b and d), a sinc pulse was used with Tp,sinc = 0.1 ms and TE = 0.19 ms.
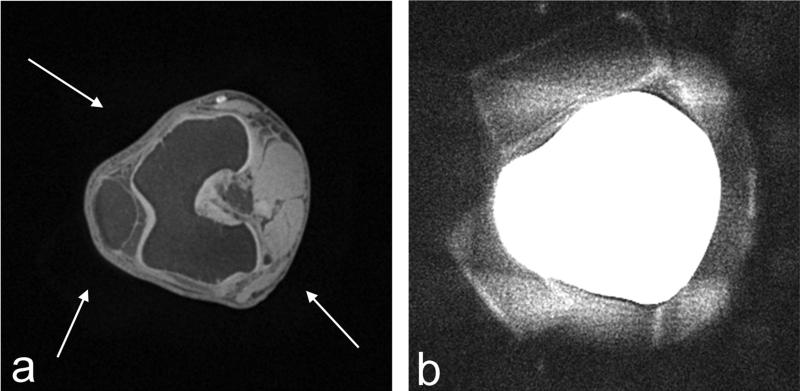
In vivo human knee imaging was also performed using CODE with the shortest TE, i.e., TE = 0.23 ms, which could be practically attained with the 3T Siemens Trio system, when FOV = 25 cm. Fat suppression was performed by applying a spectrally-selective suppression pulse after every 32 acquisitions. A sinc pulse with R = 10 was used for spin excitation. Other parameters were: Tp,sinc = 0.1 ms, flip angle = 4°, and TE = 0.23 ms. The number of projections was 100 k, yielding 0.65 mm3 isotropic resolution in a scan time of ~5 min. To illustrate the general quantity of the 3D imaging, Fig. 5a displays a CODE knee image in an axial view at the level of the patellofemoral joint, which includes anatomical structures such as the patella, femoral lateral and medial trochlear groove articular cartilage, femur, and anterior cruciate ligament insertion at the lateral femoral condyle, along the left-to-right direction. It is worthwhile to note that with a closer look some blurry shades around the knee can be recognized (Fig. 5a, white arrows) which arise from foam pads used to restrict knee movement. These pads, which are in the gap between the knee and the knee coil, are more clearly seen with slice averaging. In Fig. 5b, twenty slices were averaged around the slice shown in Fig. 5a and the averaged image was rescaled for better visualization of the foam padding. While signals from the foam padding are not usually visible with conventional MRI sequences, Fig. 5b illustrates that special attention should be paid to the choice of materials used for supporting structures within the FOV when using ultrashort TE imaging, even when using only modestly short TE of ~0.2 ms.
FIG. 5.
(a) Axial image and (b) slice-averaged and rescaled image of in vivo human knee acquired using CODE with TE = 0.23 ms. Tp,sinc = 0.1 ms, flip angle = 4°, FOV (= THK) = 25 cm, isotropic resolution = 0.65 mm3, and scan time = ~5 min. In (a), an axial knee image was chosen at the level of the patellofemoral joint among the 3D data set, showing an anatomical structure such as the patellar, the femoral lateral and medial trochlear groove articular cartilage, the femur, and the anterior cruciate ligament insertion at the lateral femoral condyle, along the left-to-right direction in the slice. With a closer look, some blurry shades around the knee can be recognized in (a), which are pointed to (white arrows). These signals arise from the foam pads placed between the knee and coil to minimize motion artifacts. These pads are more clearly seen in the image shown in (b) which was obtained by averaging 20 adjacent slices and adjusting the image intensity.
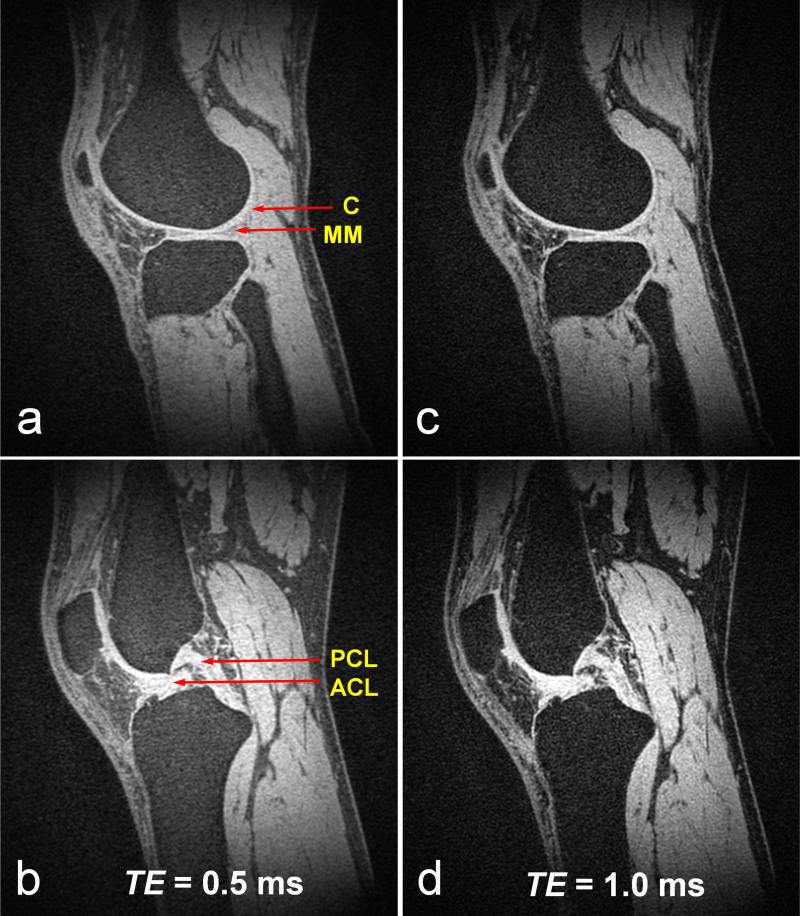
Sagittal images of in vivo human knee acquired using CODE with TE = (a) 0.5 ms and (b) 1.0 ms are shown in Fig. 6. A sinc pulse with Tp,sinc = 0.25 ms and R = 12.7 was used for spin excitation. Other parameters were: flip angle = 4°, TR = 4 ms, and FOV (= slab width) = 25 cm. The number of projections was 128 k, yielding = 0.91 mm3 isotropic resolution in a scan time of ~8 min. Fat suppression was also performed using a spectrally-selective suppression pulse after every 32 acquisitions. With both TE values, CODE provided good sensitivity to visualize connective tissues with a majority of short T2 components, such as cartilage, anterior and posterior horn medial menisci, patellar tendon, and anterior and posterior cruciate ligament. Although fat signals were generally well suppressed, signals from fatty tissues increased as TE decreased. This may be due to an increasing contribution of short T2 signal components from fatty tissue as TE decreases.
FIG. 6.
Sagittal images of in vivo human knee acquired using CODE with different echo times, i.e., TE = 0.5 ms in (a) and (b), TE = 1.0 ms in (c) and (d). Tp,sinc = 0.25 ms, flip angle = 4°. TR = 4 ms, and FOV (= slab width) = 25 cm. The number of projections = 128 k. Scan time = ~8 min. Isotropic resolution = 0.91 mm3. Fat suppression was performed using a spectral-selective suppression pulse after every 32 acquisitions. With both TE values, CODE provides good sensitivity for visualizing connective tissues with a majority of short T2 components, such as cartilage (C) (a, c), anterior and posterior horn medial menisci (MM) (a, c), patellar tendon (a ~ d), and anterior and posterior cruciate ligament (ACL and PCL) (b, d). Despite good suppression of fat signals, signals from fatty tissues increased as TE decreased, which may be due to the increasing contribution of the short T2 components of fatty tissue.
DISCUSSION AND CONCLUSIONS
The most appealing feature of the two 3D radial GRE sequences described here (rGRE and CODE) is their readiness for implementation on standard clinical MR systems, apparently without placing stringent or unique demands on hardware performance. As shown in this work, these techniques can attain relatively short TE and TR despite the limitations of peak RF power, transmit/receive switching time, coil ring down time, and slew rate that are typical for a standard 3 T clinical scanner. These radial GRE sequences can avoid some of the technical challenges arising from non-uniform sampling that conventional UTE imaging encounters, since data sampling occurs after the gradient has reached a stable plateau. An advantage of 3D MRI is the ability to obtain high-resolution images without missing any information between slices, but 3D MRI has the disadvantage of typically requiring a much longer scan time than 2D imaging. In the case of these radial imaging techniques, however, there are potential ways of reducing the scan time which were not exploited here, e.g., by under-sampling projection trajectories (16,17). In principle, with radial acquisitions, the number of projections, which determines the scan time, does not affect the spatial resolution, but rather affects the size of artifact-free zone.
The CODE sequence has the unique feature that the dephasing lobe of the readout gradient is applied during RF excitation. Such an application of the initial dephasing (or pre-dephasing) gradient during RF excitation enables CODE to offer a much shorter TE than conventional GRE methods and ~15% shorter TE than the rGRE sequence employing a short square pulse. Here it was shown that TE ≥ ~0.2 ms can be achieved in high resolution 3D imaging with a 3T clinical scanner. For example, the achievable shortest TE is 0.22 ms and 0.18 ms for FOV = 25 cm and 40 cm, respectively, as shown in Table 1. Although the theoretically expected minimum TE is 0.19 ms and 0.16 ms for FOV = 25 cm and 40 cm, respectively (Table 2), the shortest TE values that could be attained in this practical setting with a standard clinical scanner are 20 – 30 μs longer than the theoretically expected ones due to some hardware limitations and/or the automatic hardware synchronization inside the system designed to eliminate possible timing errors.
Limitations in CODE
In CODE, a frequency-selective pulse with a very short pulse duration (e.g., a sinc with Tp = 0.1 – 0.2 ms) is used for reducing TE, and thus, a flip-angle restriction is put on CODE. In other words, CODE may be unable to attain large flip angles, i.e., flip angles larger than ~10°, due to the peak B1 or SAR limitation (Table 1). Thus, although a certain degree of T1-weighting is possible, extreme T1-weighted imaging cannot be achieved in human applications with CODE when the shortest TE is desired. Of course, a larger flip angle can also be attained in CODE by increasing Tp at the cost of a longer TE. In this case, TE should be increased by the amount of increase in Tp/2 provided that other parameters remain the same.
Because a frequency-selective pulse is used for excitation in CODE, the initial dephasing gradient for readout must also serve as a slab-selective gradient during excitation. However, strictly speaking, the “real” slab selection does not work for CODE, since it is based on a radial acquisition scheme. In other words, the slab excitation inside an object along a specific direction is not currently feasible because the orientation of the slab changes with every projection. For the same reason, it is also challenging to image a selected region-of-interest (ROI) inside an object by using this slab selection. Recently, ROI reconstruction from limited-angle local projection data, which is called interior reconstruction, was proposed for computed tomography (18,19) and provided the promising possibility that it is applicable to MRI with radial sampling (20). However, the current version of CODE can only be applied to 3D MRI without limiting the FOV by slab selection, and thus, in every direction the excited slab width should exceed the dimensions of the object, unless the sensitive volume is bounded by the sensitivity profile(s) of the local receiver coil(s).
Although CODE cannot attain a TE much less than ~0.2 ms with current clinical scanners, it can be applied to a variety of musculoskeletal studies. Connective tissues, such as meniscus, tendons, ligaments, and cortical bone, contain predominantly bound water with short T2 values on the order of a few hundreds of microseconds to a few milliseconds (21-23). As demonstrated in the musculoskeletal system in this paper, CODE offers a combination of high SNR with the ability to create differential contrast between tissues dominated by short T2 components. The images reveal details such as the parallel orientation of the quadriceps tendon fiber bundles, the medial menisci, and the anterior and posterior cruciate ligament. CODE is also potentially useful for dynamic contrast enhanced (DCE) MRI studies at high magnetic fields, since it can effectively reduce T2*-weighting or signal loss which distorts the time-intensity curve and thus leads to erroneous estimates of pharmacokinetic parameters (24).
This study shows that 3D radial GRE sequences such as rGRE and CODE have the capability of permitting TE as short as ~0.2 ms using a standard clinical scanner. This range of TE is not shorter than that possible with UTE, SWIFT, and WASPI, but it might be sufficient for many types of MRI studies of short T2 signals. Considering the straightforward implementation and the apparent robustness of these 3D radial GRE sequences, they appear to be promising alternatives to other ultrashort T2 sequences in certain cases.
ACKNOWLEDGMENTS
This research was supported by NIH grant P41 RR008079. This work was also supported by National Research Foundation of Korea (NRF) funded by Ministry of Education, Science, and Technology (No. 2010-0025744).
Grant Sponsors: NIH Grants P41 RR008079, NRF Grants No. 2010-0025744
Symbols
- Gro
Italic Roman upper case ‘gee’ with subscript Roman lower cases ‘ro’
- Gss
Italic Roman upper case ‘gee’ with subscript Roman lower cases ‘ss’
- τ
Greek lower case ‘tau’
- Tp
Italic Roman upper case ‘tee’ with subscript Roman lower case ‘pee’
- Tacq
Italic Roman upper case ‘tee’ with subscript Roman lower cases ‘acq’
- THK
Italic Roman upper cases ‘THK’
- THK
Italic Roman upper cases ‘FOV’
- BW
Italic Roman upper cases ‘BW’
- SW
Italic Roman upper cases ‘SW’
- γ
Greek lower case ‘gamma’
- TECODE
Italic Roman upper cases ‘TE’ with subscript Roman upper cases ‘CODE’
- τss
Italic Greek lower case ‘tau’ with subscript Roman lower cases ‘ss’
- τro
Italic Greek lower case ‘tau’ with subscript Roman lower cases ‘ro’
- τext
Italic Greek lower case ‘tau’ with subscript Roman lower cases ‘ext’
- Tshort
Italic Roman upper case ‘tee’ with subscript Roman lower cases ‘short’
- SR
Italic Roman upper cases ‘SR’
- TE1
Italic Roman upper case ‘TE’ with subscript ‘one’
- TE2
Italic Roman upper case ‘TE’ with subscript ‘two’
- τss,1
Italic Greek lower case ‘tau’ with subscript Roman lower cases ‘ss’, coma symbol, ‘one’
- τss,2
Italic Greek lower case ‘tau’ with subscript Roman lower cases ‘ss’, coma symbol, ‘two’
- Tshort,1
Italic Roman upper case ‘tee’ with subscript Roman lower cases ‘short’, coma symbol, one’
- Tshort,2
Italic Roman upper case ‘tee’ with subscript Roman lower cases ‘short’, coma symbol, ‘two’
- BW1
Italic Roman upper case ‘BW’ with subscript ‘one’
- BW2
Italic Roman upper case ‘BW’ with subscript ‘two’
- Gss,1
Italic Roman upper case ‘gee’ with subscript Roman lower cases ‘ss’, coma symbol, ‘one’
- Gss,2
Italic Roman upper case ‘gee’ with subscript Roman lower cases ‘ss’, coma symbol, ‘two’
- TRCODE
Italic Roman upper cases ‘TR’ with subscript Roman upper cases ‘CODE’
- nacq
Italic Roman lower case ‘en’ with subscript Roman lower cases ‘acq’
- nshort
Italic Roman lower case ‘en’ with subscript Roman lower cases ‘short’
- R
Italic Roman upper cases ‘aal’
- Gdeph
Italic Roman upper case ‘gee’ with subscript Roman lower cases ‘deph’
- TErGRE
Italic Roman upper cases ‘TE’ with subscript Roman lower case ‘aal’, Roman upper cases ‘GRE’
- τdeph
Italic Greek lower case ‘tau’ with subscript Roman lower cases ‘deph’
- TRrGRE
Italic Roman upper cases ‘TR’ with subscript Roman lower case ‘aal’, Roman upper cases ‘GRE’
- B1
Italic upper case ‘bee’ with subscript ‘one’
- c
Italic Roman lower cases ‘see’
- Adeph
Italic Roman upper case ‘ei’ with subscript Roman lower cases ‘deph’
- Areph
Italic Roman upper case ‘ei’ with subscript Roman lower cases ‘reph’
- π
Greek lower case ‘pi’
- Tp,sinc
Italic Roman upper case ‘tee’ with subscript Roman lower case ‘pee’, coma symbol, Roman lower cases ‘sinc’
- Tp,square
Italic Roman upper case ‘tee’ with subscript Roman lower case ‘pee’, coma symbol, Roman lower cases ‘square’
Footnotes
Presented in part at the ISMRM meetings in Seattle, USA, 2006 and Berlin, Germany, 2007.
REFERENCES
- 1.Pauly JM, Conolly SM, Nishimura DG, Macovski A. Slice-selective excitation for very short T2 species. in 8th Annual Meeting of the International Society of Magnetic Resonance in Medicine. Amsterdam. 1989:28. [Google Scholar]
- 2.Glover GH, Pauly JM, Bradshaw KM. Boron-11 imaging with a three dimensional reconstruction method. J Magn Reson Imaging. 1992;2:47–52. doi: 10.1002/jmri.1880020109. [DOI] [PubMed] [Google Scholar]
- 3.Idiyatullin D, Corum C, Park J-Y, Garwood M. Fast and quiet MRI using a swept radiofrequency. J Magn Reson. 2006;181:342–349. doi: 10.1016/j.jmr.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Idiyatullin D, Corum C, Moeller S, Garwood M. Gapped pulses for frequency-swept MRI. J Magn Reson. 2008;193:267–273. doi: 10.1016/j.jmr.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahmer J, Bornert P, Groen J, Bos C. Three-dimensional radial ultrashort echo-time imaging with T2 adapted sampling. Magn Reson Med. 2006;55:1075–1082. doi: 10.1002/mrm.20868. [DOI] [PubMed] [Google Scholar]
- 6.Brittain JH, Shankaranarayanan A, Ramanan AV, Shimakawa A, Cunningham CH, Hinks RS, Francis IR, Turner R, Johnson J, Nayak KS, Tan SG, Pauly JM, Bydder GM. Ultrashort TE imaging with single-digit (8μs) TE. in 12th Annual Meeting of the International Society of Magnetic Resonance in Medicine. Kyoto. 2004:629. [Google Scholar]
- 7.Tyler DJ, Robson MD, Henkelman RM, Young IR, Bydder GM. Magnetic resonance imaging with ultrashort TE (UTE) pulse sequences: Technical considerations. J Magn Reson Imaging. 2007;25:279–289. doi: 10.1002/jmri.20851. [DOI] [PubMed] [Google Scholar]
- 8.Wu Y, Ackerman JL, Chesler DA, Graham L, Wang Y, Glimcher MJ. Density of organic matrix of native mineralized bone measured by water- and fat-suppressed proton projection. MRI Magn Reson Med. 2003;50:59–68. doi: 10.1002/mrm.10512. [DOI] [PubMed] [Google Scholar]
- 9.Madio DP, Lowe IJ. Ultra-fast imaging suing low flip angles and FIDs. Magn Reson Med. 1995;34:525–529. doi: 10.1002/mrm.1910340407. [DOI] [PubMed] [Google Scholar]
- 10.Tsao J, Behnia B, Webb AG. Unifying linear prior-information-driven methods for accelerated image acquisition. Magn Reson Med. 2001;46:652–660. doi: 10.1002/mrm.1242. [DOI] [PubMed] [Google Scholar]
- 11.Kuethe DO, Caprihan A, Lowe IJ, Madio DP, Gach MH. Transforming NMR data despite missing points. J Magn Reson. 1999;139:18–25. doi: 10.1006/jmre.1999.1767. [DOI] [PubMed] [Google Scholar]
- 12.Hsu J-J, Lowe IJ. Signal recovery in free induction decay imaging using a stimulated echo. Magn Reson Med. 2002;47:409–414. doi: 10.1002/mrm.10057. [DOI] [PubMed] [Google Scholar]
- 13.Kuethe DO, Adolphi NL, Fukushima E. Short data-acquisition times improve projection images of lung tissue. Magn Reson Med. 2007;57:1058–1064. doi: 10.1002/mrm.21230. [DOI] [PubMed] [Google Scholar]
- 14.Wong STS, Roos MS. A strategy for sampling on a sphere applied to 3D selective RF pulse design. Magn Reson Med. 1994;32:778–784. doi: 10.1002/mrm.1910320614. [DOI] [PubMed] [Google Scholar]
- 15.Pipe JG, Menon P. Sampling density compensation in MRI: Rationale and an iterative numerical solution. Magn Reson Med. 1999;41:179–186. doi: 10.1002/(sici)1522-2594(199901)41:1<179::aid-mrm25>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 16.Peters DC, Korosec FR, Grist TM, Block WF, Holden JE, Vigen KK, Mistretta CA. Undersampled projection reconstruction applied to MR angiography. Magn Reson Med. 2000;43:91–101. doi: 10.1002/(sici)1522-2594(200001)43:1<91::aid-mrm11>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Barger AV, Block WF, Toropov Y, Grist TM, Mistretta CA. Time-resolved contrast-enhanced imaging with isotropic resolution and broad coverage using an undersampled 3D projection trajectory. Magn Reson Med. 2002;48:297–305. doi: 10.1002/mrm.10212. [DOI] [PubMed] [Google Scholar]
- 18.Ye Y, Yu H, Wang G. Exact interior reconstruction with cone-beam CT. Int J Biomed Eng. 2007 doi: 10.1155/2007/10693. ID 10693:5 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ye Y, Yu H, Wang G. Exact interior reconstruction from truncated limited-angle projection data. Int J Biomed Eng. 2008 doi: 10.1155/2008/427989. ID 427989:6 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Yu H, Corum C, Garwood M, Wang Ge. Exact and stable interior ROI reconstruction for radial MRI. Medical Imaging 2009: Physics of Medical imaging. Proc. of SPIE. 7258:72585G. [Google Scholar]
- 21.Gold GE, Thedens DR, Pauly JM, Fechner KP, Bergman G, Beaulieu CF, Macovski A. MR imaging of articular cartilage of the knee: new methods using ultrashort TEs. Am J Roentgenol. 1998;170:1223–1226. doi: 10.2214/ajr.170.5.9574589. [DOI] [PubMed] [Google Scholar]
- 22.Gatehouse PD, Thomas RW, Robson MD, Hamilton G, Herlihy AH, Bydder GM. Magnetic resonance imaging of the knee with ultrashort TE pulse sequences. Magn Reson Img. 2004;22:1061–1067. doi: 10.1016/j.mri.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Reichert ILH, Robson MD, Gatehouse PD, He T, Chappell KE, Holmes J, Girgis S, Bydder G. Magnetic resonance imaging of cortical bone with ultrashort TE pulse sequences. Magn Reson Img. 2005;23:611–618. doi: 10.1016/j.mri.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 24.de Bazelaire C, Rofsky NM, Duhamel G, Zhang J, Michaelson MD. Combined T2* and T1 measurements for improved perfusion and permeability studies in high field using dynamic contrast enhancement. Eur Radiol. 2006;16:2083–2091. doi: 10.1007/s00330-006-0198-1. [DOI] [PubMed] [Google Scholar]