Figure 1.
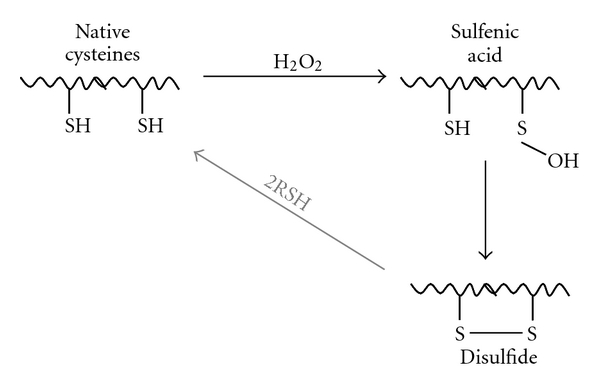
Formation of intramolecular disulfide bonds (S–S). Oxidation of a cysteine thiol by H2O2 yields a sulfenic acid residue that can undergo reaction with a neighbouring “back door” cysteine thiol to generate a disulfide linkage (S–S). S–S bonds can overtime be returned to the native SH state by reactions with biological thiols (RSH). This picture was adapted from [21].
