Abstract
β-adrenergic signaling is involved in the development of cardiac hypertrophy (CH), justifying the use of β-blockers as a therapy to minimize and postpone the consequences of this disease. Evidence suggests that adenylate cyclase, a downstream effector of the β-adrenergic pathway, might be a therapeutic target. We examined the effects of the anti-epileptic drug carbamazepine (CBZ), an inhibitor of adenylate cyclase. In a murine cardiac hypertrophy model, carbamazepine significantly attenuates isoproteronol (ISO)-induced cardiac hypertrophy. Carbamazepine also has an effect in transverse aortic banding induced cardiac hypertrophy (TAB) (P=0.07). When carbamazepine was given in combination with the antibiotic doxycycline (DOX), which inhibits matrix metalloproteinases (MMPs), therapeutic outcome measured by heart weight-to-body weight and heart weight-to-tibia length ratios was improved compared to either drug alone. Additionally, the combination therapy resulted in an increase in the survival rate over a 56-day period compared to that of untreated mice with cardiac hypertrophy or either drug used alone. Moreover, in support of a role for carbamaze -pine as a β-adrenergic antagonist via cAMP inhibition, a lower heart rate and a lower level of the activated phosphorylated form of the cAMP Response Element-Binding (CREB) were observed in heart extracts from mice treated with carbamazepine. Gene expression analysis identified 19 genes whose expression is significantly altered in treated animals and might be responsible for the added benefit provided by the combination therapy. These results suggest that carbamazepine acts as a β-adrenergic antagonist. Carbamazepine and doxycycline are approved by the US Food and Drug Administration (FDA) as drugs that might complement medications for cardiac hypertrophy or serve as an alternative therapy to traditional β-blockers. Furthermore, these agents reproducibly impact the expression of genes that may serve as additional therapeutic targets in the management of cardiac hypertrophy.
Key words: cardiac hypertrophy, gene expression, drug repurposing, FDA approved.
Introduction
Cardiac hypertrophy (CH) frequently develops in response to biomechanical stress or increased cardiac afterload, such as in the setting of systolic hypertension or aortic stenosis. Hypertrophic hearts often develop contractile dysfunction and decreased cardiac performance, with an increased risk for heart failure, ischemic heart disease, and sudden death.1,2 Cardiac hypertrophy remains a chronic and progressive process even though a number of generally-acceptable medication therapies are available, such as angiotensin converting enzyme (ACE) inhibitors and beta blockers. More effective therapies are still needed to prevent heart deterioration in individuals with hypertrophy and to reduce unwanted side effects associated with current medications.
The process of cardiac hypertrophy development is complex, and there are multiple molecular signaling pathways contributing to the hypertrophic response triggered by mechanical stress.3–5 Perhaps the best characterized is the β-adrenergic pathway, which is a major hypertrophic stimulus mediated via a G protein-coupled receptor that activates adenylate cyclase and subsequently cAMP production. Isoproterenol (ISO), a β-adrenergic agonist that induces cardiac hypertrophy in mice, has been previously shown to increase cAMP production in cultured myocytes, comparable to forskolininduced cAMP levels.6 Similarly, disruption of the gene encoding adenylate cyclase has been shown to prevent ISO- or pressure overload-induced cardiac hypertrophy.7 Moreover, β-blockers are well established as therapies that counter the consequences of hypertension and hypertrophy by preventing stimulation of this pathway and subsequently improving the survival rates of patients suffering from hypertrophy or heart failure.8–10 To discover potential new therapies for cardiac hypertrophy, we used IRIDESCENT, a computational program that can identify previously unknown relationships between medical objects in PubMed such as small molecules, phenotypes, and genes.11 This novel method of data mining has been shown to be a useful tool for identifying potential drug candidates and previously predicted the known relationship between chlorpromazine and cardiac hypertrophy.12 IRIDESCENT predicted several other therapeutic candidates which were further screened by examining their published modes of action and potential for targeting pathways known to be important for cardiac hypertrophy. One candidate was the antibiotic doxycycline (DOX) which inhibits MMPs. We have previously shown that doxycycline attenuates the hypertrophic phenotype in mice challenged with isoproterenol or transverse aortic banding and that doxycycline was able to reduce potential signaling events associated with cardiac hypertrophy, including MMP2/9 activation, upregulation of EDG1 receptor and activation of ERK, p-38, and the transcription factor ATF-2.13 Another candidate selected from IRIDESCENT predictions is the anti-epileptic carbamazepine. Carbamazepine has been shown to decrease both basal and forskolin-stimulated cAMP production by inhibiting adenylate cyclase and its downstream effects.14,15,16 In this study, we tested the efficacy of both carbamazepine and doxycycline in a mouse model of cardiac hypertrophy and found that carbamazepine significantly attenuated hypertrophy. When carbamazepine and doxycycline were administered in combination, the hypertrophic phenotype was further decreased and survival increased. Our results suggest that carbamazepine mediates these beneficial effects by interfering with β-adrenergic signaling.
To further define the specific molecular signaling events that might be altered by doxycycline and carbamazepine treatment, we also performed hybridization microarray analysis to measure differential gene expression and to identify other potential molecular targets for the management of cardiac hypertrophy.
Materials and Methods
Isoproterenol induced cardiac hypertrophy
All animal studies were conducted in accordance with the standards set forth in published guidelines17 and were approved by our Institutional Animal Care and Use Committee. Eight-week-old C57BL/6J male mice (Jackson Laboratory) were given isoproterenol (Sigma Aldrich) at 40 mg.kg−1.d−1 administered S.Q. via micro-osmotic pump insertion (ALZET 1007D). Briefly, animals were anesthetized with 1.5% isoflurane and 98.5% oxygen using an animal ventilator (Surgivet), a 1 cm incision was made on the back between the scapulae, and micro-osmotic pumps containing isoproterenol dissolved in a saline solution (0.9% NaCl) were inserted into the infrascapular subcutaneous tissue.
Transverse aortic banding-induced cardiac hypertrophy
Increased pressure in the proximal aorta was induced by means of transverse aortic banding (TAB), as previously described.18 Male mice (C57Bl6, eight weeks old) were anesthetized with ketamine (100 mg/kg IP) plus xylazine (5 mg/kg IP), orally intubated with 20-gauge tubing, and ventilated (Harvard Apparatus Rodent Ventilator, model 687) at 120 breaths per minute (0.1-mL tidal volume). A 3-mm left-sided thoracotomy was performed at the second intercostal space. The transverse aortic arch was ligated (7-0 Prolene) between the innominate and left common carotid arteries with an overlying 27-gauge needle, and then the needle was removed, leaving a discrete region of stenosis. The chest was closed, and the left-sided pneumothorax was evacuated. Perioperative (24 hours) and one-week mortalities were less than 10% each. Mice were sacrificed after 21 days of experimentation.
Heart rates
Non-invasive recording of heart rate (HR) and electrocardiogram (ECG) were accomplished in conscious mice using the AnonyMouse™ ECG Screening System (Mouse Specifics, Inc., Boston, MA, USA).19 Mice were rested (i.e. not manipulated) for at least 10 min before readings were recorded. For each mouse, a reading was obtained by averaging the first three readings of more than 5 sec.
Administration of doxycycline and carbamazepine
Doxycycline (Sigma Aldrich) was given at 6 mg/mL in drinking water containing 5% sucrose. Control animals were given 5% sucrose water. Carbamazepine was administered in rodent chow by mixing powdered chow with 0.25% carbamazepine. Water was added to the dry chow mix at the ratio 0.8:1 water:powder by weight and the resulting paste diced and dried overnight at 60°C. The half-life for doxycycline is shortened when coadministered with carbamazepine.20 Therefore we increased the concentration of doxycycline to 10 mg/mL in 7% sucrose water in co-administration experiments. Control animals in coadministration experiments received 7% sucrose in water.
Microarray sample preparation and analysis
Mice were sacrificed by cervical dislocation after the animals were anesthetized with 3:97 isoflurane and oxygen mix. Animals' hearts were then rapidly removed and the blood flushed out with 3 mL of 0.9% saline solution. The atria and right ventricles were cut from the heart and immediately plunged into TRIzol Reagent (Life Technologies). Total RNA was isolated following the manufacturer's instructions, purified by phenol-chloroform extraction and ethanol precipitation, and 20 µg further processed for microarray analysis. Briefly, cDNA synthesis, in vitro transcription, and labeling and fragmentation to produce the oligonucleotide probes were performed as instructed by the microarray manufacturer. The probes were first hybridized to a test array (Affymetrix) and then to the GeneChip Mouse Genome 430 2.0 Array (Affymetrix), using the GeneChip Hybridization Oven 640 (Affymetrix). The chips were washed in a GeneChip Fluidics Station 450 (Affymetrix), and the results were visualized with a GeneChip G7 scanner (Affymetrix). One mouse heart was used for each array, and the experiment was performed in triplicate for four conditions (ISO, ISO + DOX, ISO + CBZ, ISO + DOX + CBZ) generating a total of 12 arrays. GeneSifter (VizX Labs, Seattle, WA, USA) was used to perform RMA normalization, pairwise comparisons of averaged signal intensity values, and Student's t-test with Benjamini and Hochberg correction, and Spotfire DecisionSite 8.3 (Spotfire, Inc., Somerville, MA) was used to perform pairwise comparisons of the individual experiments. A gene was considered significantly altered in expression if the average fold-change value was at least 2.0, the fold change for each individual replicate comparison was at least 1.5, and the corrected P value was less than 0.05. Additionally, genes that were altered between any two normal or any two ISO-treated samples were removed, as these alterations most likely represented normal variations between mice.
Real-Time reverse transcriptase-polymerase chain reaction
Quantitative RT-PCR was performed in the iCycler iQ multi-Color real-time PCR detection system (Bio-Rad, Hercules, CA, USA) using SYBR Green I dye (Qiagen, Valencia, CA, USA) as described by the manufacturer. Briefly, 100 ng of RNA was placed into a 25 µL reaction volume containing 2.5 µL of each primer set (Quantitect Primer Assays, Qiagen), 12.5 µL SYBER Green PCR master mix, and 0.25 µL reverse transcriptase. A typical protocol included reverse transcription at 50°C for 30 min and a denaturation step at 95°C for 15 min followed by 35 cycles with 94°C denaturation for 15 sec, 55°C annealing for 30 sec, and 72°C extension for 30 sec. Detection of the fluorescent product was performed at the end of the extension period at 60°C for 20 sec. The PCR products were subjected to a melting curve analysis to confirm amplification specificity. Negative controls containing water instead of RNA were run concurrently to confirm that the samples were not cross-contaminated. Targets were normalized to reactions performed using Quantitect GAPDH primer assay (Qiagen), and fold change was determined using the comparative threshold method.21
Histology
Animal hearts were excised and fixed with 10% phosphate-buffered formalin for 48h, and then embedded in paraffin. Cross-sectional slices in the minor axis were obtained with a microtome and the slices stained using Mayer's hematoxylin and eosin (H&E).
Immunoblotting
Left ventricles were homogenized in 100 mM Tris-HCl (pH 7.4) containing 15% glycerol, 2 mM EDTA, 2% SDS, and 0.1 mM phenylmethylsulfonylfluoride. Homogenates were heated at 95°C for 10 min, passed through a 23-gauge needle five times, and centrifuged at 12,000g for 10 min.22 Proteins (80 µg/mL) were resolved by 10% SDS-PAGE and electrophoretically transferred onto a nitrocellulose membrane (Amersham Hybond, GE Healthcare, NJ, USA). Membranes were blocked with 5% milk (Bio-Rad, CA, USA) and probed with affinity-purified antibodies at 1:1000 dilution. Membranes were subsequently incubated with horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology, MA, USA) and then exposed to chemiluminescence substrate (GE Healthcare). Affinity-purified anti-CREB-P and anti-GAPDH antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Band intensities and sizes were obtained using Adobe Photoshop CS3 (Adobe Systems Inc, CA, USA).
Statistical analysis
Values presented are expressed as mean ± S.E.M. All comparisons between groups were performed using a one-way ANOVA followed by the Newman-Keuls test. Differences were considered to be statistically significant when P<0.05.
Results
Carbamazepine is beneficial in cardiac hypertrophy
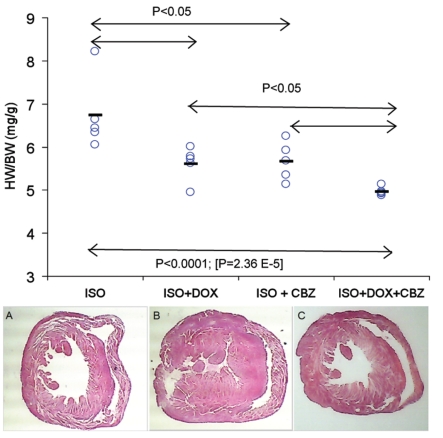
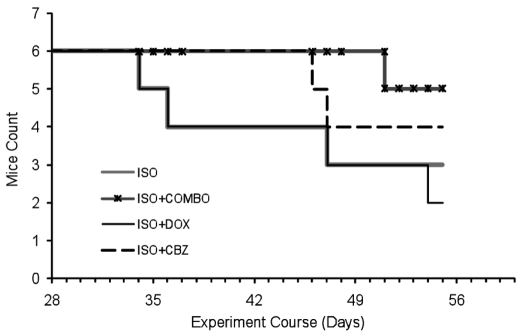
In the isoproterenol induced cardiac hypertrophy model, the untreated mouse exhibits severe hypertrophy while the carbamazepine treated heart has a structure that is relatively well preserved, suggesting that carbamazepine treatment reduced ISO-induced hypertrophy (Figure 1). Carbamazepine significantly decreased the heart weight to tibia length (HW/TL) ratio (7.14 mg/mm vs. 9.92 mg/mm, P<0.0001, n=10 mice per group) and heart weight to body weight (HW/BW) ratio (5.7 mg/g vs. 6.8 mg/g, P<0.01, n=5 mice per group; Figure 1). In the TAB model, carbamazepine also seems to attenuate cardiac hypertrophy as the HW/BW were 6.02 mg/g versus 5.61 mg/g for the TAB group and the TAB+CBZ group, respectively (n= 5 mice per group). However the P value is slightly above the significance threshold (P=0.07) suggesting that carbamazepine likely has an effect but not as visible in the case of the TAB model. The HW/BW ratios show that co-administration of doxycycline and carbamazepine together reduces hypertrophy more than either drug administered alone. This partially additive effect of the two drugs strongly supports the hypothesis that the two drugs act via different CH-associated pathways and that targeting both pathways simultaneously resulted in better therapeutic performance in the mouse model. Figure 2 shows that this benefit resulted in a substantial increase in survival time.
Figure 1.
(Top panel) The combination of DOX+CBZ provides added therapeutic benefit relative to either drug alone in mice with ISO-induced cardiac hypertrophy. P values are obtained from a one-way ANOVA. The experiment lasted 10 days. DOX was given at 10 mg/mL in 7% sucrose water. CBZ was given in chow at 0.25%. The control group (ISO only) received regular chow and 7% sucrose water. Each circle is the heart to body weight ratio for one mouse and the dashes are the average for each group. (Bottom panel) Histological cross sections of mouse hearts of CBZ-treated and untreated mice. (A) Wild type control mouse (C57BL/6J). Heart weight (HW) = 0.1305 g; body weight (BW) = 26.3g. (B) ISO-treated CBZ-untreated mouse. HW=0.1800g; BW=26.3g. (C) ISO- and CBZ-treated mouse. HW=0.1415, BW=26.3.
Figure 2.
The combination of DOX + CBZ reduces hypertrophy and increases survival relative to ISO treatment alone. There were 6 mice in each group. One mouse was sacrificed on day 7 in each group.
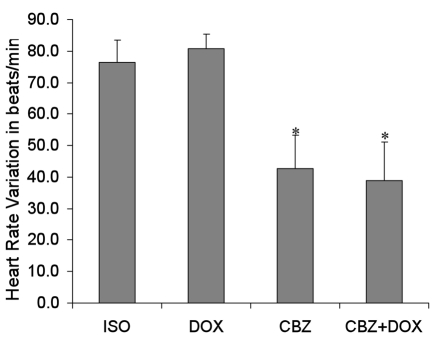
We also measured the heart rates of the mice before induction of cardiac hypertrophy and after nine days of treatment. As expected, the β-adrenergic agonist isoproterenol caused an observable increase in heart rate for each mouse compared to measurements taken prior to isoproterenol treatment (Figure 3). The maximum heart rate was 821 beats per minute for the isoproterenol group versus 780 for the cardiac hypertrophy group treated with CBZ + DOX (P<0.01, n=5). A one-way ANOVA followed by a Newman-Keuls test P value for heart rate variations is 0.007 and indicates statistically significant differences between groups of mice classified as follows: ISO=DOX>CBZ=(CBZ+DOX) (P<0.01, n=5), which indicates that attenuation of the increased heart rate induced by isoproterenol is mediated by carbamazepine. Further, since no change in heart rate was observed between ISO- and ISO + DOX-treated mice, the mechanism of action of doxycycline is likely independent of the β-adrenergic pathway.
Figure 3.
HR variation over the course of the experiment (average+SEM) of mice receiving ISO, or ISO+DOX, ISO+CBZ, or ISO+DOX+CBZ. HRs were measured at the beginning of the experiment and one day before the sacrifice. Each HR is the average of 3 measurements. The experiment lasted ten days. The one-way ANOVA P value is 0.007 and indicates differences in groups (n=5). A Newman-Keuls test led to the conclusion that groups can be classified as follows: ISO=DOX>CBZ= (CBZ+DOX. *Indicates significant differences.
Effects on gene expression profile
To assess the effect of doxycycline and carbamazepine on cardiac gene expression, microarray analyses were performed on normal (no drug) mice (N) and mice with ISOinduced cardiac hypertrophy that were subsequently untreated (CH) or treated with DOX, CBZ, or DOX + CBZ. Based on various selection criteria (see Materials and Methods), there were 779 genes that were significantly altered between N and CH mice. Of these 779 genes, 327 and 472 were altered in the reverse direction when mice were given doxycycline or the combination drug treatment, respectively. Only one gene was significantly altered, based on the stringent analysis criteria used, in mice treated with carbamazepine alone. This gene (G7e protein) encodes a viral capsid protein of otherwise unknown function (−2.2-fold).
Of the 472 “CH-specific” genes that were altered in response to treatment with DOX + CBZ, 453 and 98 were also altered when either doxycycline or carbamazepine alone was used, when statistical parameters were lifted (i.e., average fold changes irrespective of statistical measures). The remaining 19 genes were only altered in mice given isoproterenol, compared to normal mice, and in mice given the doxycycline + carbamazepine drug therapy (in the opposite direction), but not when either drug was administered alone. Presumably these 19 genes represented transcriptional alterations observed that correlate with the additive effect of both drugs. Functions of these genes included those involved in transport processes, cytoskeleton movement/adhesion, and muscle/heart development. Eighteen of the gene alterations that were determined to be differentially expressed between disease conditions were verified by real-time RT-PCR (Table 1).
Table 1. Real-time RT-PCR results for selected genes.
| Microarray | Real-time RT-PCR | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene name | Function | FC | |||||||
| CH | Combo | DOX | CBZ | CH | Combo | DOX | CBZ | ||
| DNA-damage-inducible transcript 4 (Ddit4) | Hypoxic stress response; cell growth | 7.4 | −6.2 | −5.6 | - | 13.9 | −2.5 | −5.7 | - |
| Matrix metalloproteinase 3 (Mmp3) | Extracellular matrix remodeling | 7.4 | −3.6 | −2.9 | - | 5.3 | −1.5 | −3.5 | −2.5 |
| Metallothionein 1 (MT2) | NO-mediated signal transduction | 15.6 | −6.8 | −5.5 | - | 19.7 | −2.8 | −14.9 | - |
| Tubulin, alpha 4 (Tuba4) | Microtubule-based movement | −6.2 | 5.8 | 5.8 | - | −26.0 | 27.9 | 36.8 | - |
| GATA binding protein 4 (Gata4) | Transcription regulation; heart development | −3.0 | 4.0 | 2.7 | 2.1 | −3.3 | 8.6 | 4.9 | 2.0 |
| Serine protease inhibitor 2-2(Spi2-2) (Serpin3n) | Acute-phase response; inflammation | 40.5 | −12.7 | −9.7 | −2.3 | 90.5 | −18.4 | −3.8 | −13.9 |
| Transformation related protein 53 inducible nuclear protein 1 (Trp53inp1) | Stress response; apoptosis | 4.8 | −5.0 | −3.5 | - | 6.1 | Red | −1.9 | −3.0 |
| NADPH oxidase 4 (Nox4) | Electron transport; superoxide release | 4.3 | −3.4 | −3.2 | −1.9 | 22.6 | −2.5 | −4.0 | −3.5 |
| Gem GTPase (Gem) | Calcium channel blockage | 2.7 | −2.9 | −2.8 | - | 8.0 | −1.7 | −1.8 | - |
| Oncostatin receptor (Osmr) | Inflammation; connective tissue production; extracellular matrix turnover | 5.1 | −2.9 | −2.6 | −1.7 | Ind | Red | Red | Red |
| Phospholipase A2 group VII (platelet-activating factor acetylhydrolase, plasma) (Pla2g7) | Inflammation; lipid catabolism | 3.7 | −2.3 | −2.2 | - | 7.5 | −2.0 | −1.9 | −3.5 |
| SET and MYND domain containing 1 (Smyd1) | Heart development | −2.9 | 3.2 | 2.7 | 1.7 | −1.7 | 4.9 | 1.6 | 3.3 |
| Lipocalin 2 (Lcn2) | Vascular remodeling; apoptosis | 27.7 | −16.6 | −13.3 | −1.6 | 64.0 | −7.0 | −9.9 | - |
| Cyclin-dependent kinase inhibitor 1A (p21) (Cdkn1a) | Cell cycle arrest | 14.6 | −8.6 | −6.5 | - | 128.0 | −13.0 | −7.0 | −2.5 |
| S100 calcium binding protein A8 (calgranulin A) (S100a8) | Cell proliferation; calcium signaling | 7.1 | −14.3 | −19.9 | - | 5.7 | −137.2 | −181.0 | −2.1 |
| S100 calcium binding protein A9 (calgranulin B) (S100a9) | Cell proliferation; calcium signaling | 7.1 | −15.9 | −14.0 | - | 17.2 | −52.0 | −104.0 | - |
| Cyclin G2 (Ccng2) | Cell cycle regulation | 3.4 | −3.8 | −3.1 | - | 9.2 | −2.6 | −5.7 | - |
| Cytokine inducible SH2-containing protein 3 (Socs3) | Regulation of cell growth; negative regulation of insulin signaling | 8.2 | −7.9 | −4.9 | −2.4 | 9.9 | −4.0 | −4.0 | −4.3 |
N: normal mice; CH: ISO-treated mice; DOX: mice treated with ISO + DOX; CBZ: mice treated with ISO + CBZ, and Combo: mice treated with ISO + DOX + CBZ. FC: fold change. Ind/Red (Induced/reduced) are used instead of fold changes where no transcript was detected in one of the two samples being compared.
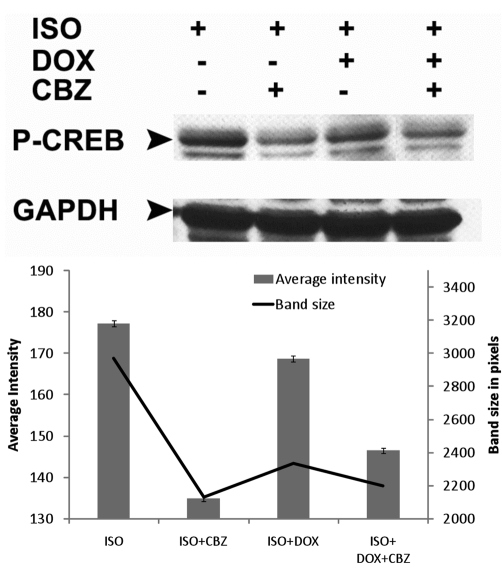
Effects on CREB phosphorylation
Carbamazepine attenuates the ISO-induced phosphorylation of CREB (Figure 4) confirming the anti-adenylate cyclase activity of carbamazepine.14 This attenuation is likely responsible for the anti-chronotropic effect of carbamazepine in mice receiving isoproterenol (Figure 3). Although doxycycline administration results in a limited attenuation of CREB phosphorylation, it may likely be a consequence of doxycycline anti-hypertrophic activity. Furthermore and unlike carbamazepine, doxycycline had no significant action on the isoproterenol induced chronotropic effect (Figure 3).
Figure 4.
Both CBZ and CBZ+DOX attenuate the ISO-induced phosphorylation of CREB. (Top panel) Western immunoblot from heart protein extracts showing levels of P-CREB. (Bottom panel) Band intensity and size quantification.
Discussion
The results of this study suggest that carbamazepine attenuates ISO-induced cardiac hypertrophy, an effect that is even more pronounced when it is administered concurrently with doxycycline. Further, the beneficial effects of the DOX + CBZ combination therapy included an increased survival rate and decreased heart rate, the latter of which was also observed when carbamazepine was administered alone. Because one of the major actions of isoproterenol is an increase in heart rate, presumably via stimulation of the β-adrenergic pathway, this study suggests that carbamazepine interferes with β-adrenergic signaling. The β-adrenergic stimulation is mediated via a G protein, coupled to adenylate cyclase. Because carbamazepine has been previously shown to inhibit adenylate cyclase,14 it is reasonable to assume that this has indeed occurred in our system as well, especially given the decreased hypertrophy and lower heart rate observed in carbamazepine treated animals compared to mice treated with isoproterenol alone.
Additionally, carbamazepine was found to have a more visible action in the ISO-induced model in comparison with the TAB model. This is likely due to the different mechanisms involved in the induction of hypertrophy in these models. The isoproterenol model is primarily mediated via β-adrenergic stimulation on the heart, while the TAB model likely involves many different pathways. Assuming that carbamazepine acts as a β-adrenergic antagonist (via the AC inhibition), it is not surprising that the carbamazepine effect is more visible in the ISO-induced model.
The likely mechanism of action of doxycycline in the context of cardiac hypertrophy is the inhibition of matrix metalloproteinases (MMPs), which are known to contribute to the hypertrophic phenotype.13 There is no reason to believe that doxycycline might exert a negative effect on β-adrenergic signaling, especially since no decrease in heart rate was observed in response to doxycycline treatment. This is consistent with previous work, in which non-selective inhibition of MMPs and knock out of specific MMP genes failed to alter blood pressure or heart rate in mice.23–25 Carbamazepine, in contrast, has been correlated with lower blood pressure and heart rates in epileptic patients26–28 and has no toxic cardiovascular effects.29 The observation that carbamazepine counters the positive chronotropic effect induced by isoproterenol via depression of β-adrenergic signaling is in accordance with previous work14 and our hypothesis that carbamazepine inhibits adenylate cyclase in cardiomyocytes in vivo.
While carbamazepine was clearly beneficial to mice after induction of cardiac hypertrophy, there was very little transcriptional alteration in carbamazepine-treated animals compared to those treated with doxycycline alone or with the drug combination. It is possible that the mechanism of action for carbamazepine lies in its ability to activate and/or inhibit cardiac hypertrophy-specific proteins post-transcriptionally and perhaps act additively with doxycycline by targeting those proteins that are increased at the level of transcription by doxycycline treatment. Regardless of how this might be accomplished, however, several cardiac-related genes were altered by these two drugs when administered alone and/or in combination. For instance, the gene that encodes cAMP-specific phosphodiesterase 4A (PDE4A ), which inactivates cAMP, was decreased in response to isoproterenol treatment and restored in response to drug therapy. It was also possible to correlate 19 genes with the added benefits observed during the combination therapy (DOX + CBZ). These genes may serve as research tools to uncover the underlying molecular mechanisms leading to cardiac hypertrophy. Interestingly, one of these 19 genes is the α-adrenergic receptor (Adra1b), which has been recently demonstrated to prevent a maladaptive cardiac response. This gene was down-regulated in isoproterenol mice and completely restored to basal levels after treatment with the DOX + CBZ combination (2.3-fold).
Additionally, carbamazepine has also been shown to inhibit histone deacetylase (HDAC),30 transcriptional modulators of genes involved in the hypertrophic response. Increasing evidence demonstrates that inhibition of HDACs, particularly of class II (preferentially expressed in the heart31) but also class I might be an efficient therapeutic strategy.32–34 Therefore, we cannot exclude the HDAC inhibition potential of carbamazepine as a rational explanation of its beneficial effect nor can we exclude the involvement of both pathways in the therapeutic effect of carbamazepine.
Since carbamazepine and doxycycline are both already FDA approved for use as an antiepileptic (CBZ) and antibiotic (DOX), the two drugs may be attractive candidates for therapies that complement currently accepted medications used to treat and prevent the disastrous effects of prolonged long-term hypertension. In epileptic patients, carbamazepine may cause problematic side effects such as fluid retention and aggravation of hyponatremia when combined with thiazides. As such, caution should be used when considering off-label prescription of carbamazepine and doxycycline to prevent any adverse effect. Additional experiments to assess the safety and efficacy of these drugs for cardiac hypertrophy are warranted.
Acknowledgments
the authors wish to thank Angela George, Robin Frink, Charles German and Joe Steninger for excellent technical help, Dr Wayne Fisher for discussions and comments and Linda Gunn for administrative assistance. This work was supported by the P.O'B. Montgomery Distinguished Chair (HG), the Hudson Foundation (HG) and National Institute of Health cardiology fellowship (CLG).
References
- 1.Kannel WB, Gordon T, Offutt D. Left ventricular hypertrophy by electrocardiogram. Prevalence, incidence, and mortality in the Framingham study. Ann Intern Med. 1969;71:89–105. doi: 10.7326/0003-4819-71-1-89. [DOI] [PubMed] [Google Scholar]
- 2.Levy D, Garrison RJ, Savage DD, et al. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N Engl J Med. 1990;322:1561–6. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 3.Chien KR. Stress pathways and heart failure. Cell. 1999;98:555–8. doi: 10.1016/s0092-8674(00)80043-4. [DOI] [PubMed] [Google Scholar]
- 4.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 5.Lips DJ, deWindt LJ, van Kraaij DJW, Doevendans PA. Molecular determinants of myocardial hypertrophy and failure: alternative pathways for beneficial and maladaptive hypertrophy. Eur Heart J. 2003;24:883–96. doi: 10.1016/s0195-668x(02)00829-1. [DOI] [PubMed] [Google Scholar]
- 6.Cui H, Green RD. Regulation of the campelevating effects of isoproterenol and forskolin in cardiac myocytes by treatments that cause increases in camp. Biochem Biophys Res Commun. 2003;307:119–26. doi: 10.1016/s0006-291x(03)01130-6. [DOI] [PubMed] [Google Scholar]
- 7.Okumura S, Takagi G, Kawabe J, et al. Disruption of type 5 adenylyl cyclase gene preserves cardiac function against pressure overload. Proc Natl Acad Sci USA. 2003;100:9986–90. doi: 10.1073/pnas.1733772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldstein S, Kennedy HL, Hall C, et al. Metoprolol cr/xl in patients with heart failure: a pilot study examining the tolerability, safety, and effect on left ventricular ejection fraction. Am Heart J. 1999;138:1158–65. doi: 10.1016/s0002-8703(99)70083-9. [DOI] [PubMed] [Google Scholar]
- 9.Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–55. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 10.Poole-Wilson PA. The cardiac insufficiency bisoprolol study ii. Lancet. 1999;353:1360–1. doi: 10.1016/S0140-6736(05)74354-3. [DOI] [PubMed] [Google Scholar]
- 11.Wren JD, Garner HR. Shared relationship analysis: ranking set cohesion and commonalities within a literature-derived relationship network. Bioinformatics. 2004;20:191–8. doi: 10.1093/bioinformatics/btg390. [DOI] [PubMed] [Google Scholar]
- 12.Wren JD, Bekeredjian R, Stewart JA, et al. Knowledge discovery by automated identification and ranking of implicit relationships. Bioinformatics. 2004;20:389–98. doi: 10.1093/bioinformatics/btg421. [DOI] [PubMed] [Google Scholar]
- 13.Errami M, Galindo CL, Tassa AT, et al. Doxycycline attenuates isoproterenol- and transverse aortic banding-induced cardiac hypertrophy in mice. J Pharmacol Exp Ther. 2008;324:1196–203. doi: 10.1124/jpet.107.133975. [DOI] [PubMed] [Google Scholar]
- 14.Chen G, et al. Attenuation of cyclic amp production by carbamazepine. J Neuro-chem. 1996;67:2079–86. doi: 10.1046/j.1471-4159.1996.67052079.x. [DOI] [PubMed] [Google Scholar]
- 15.Mathew J, Sleight P, Lonn E, et al. Reduction of cardiovascular risk by regression of electrocardiographic markers of left ventricular hypertrophy by the angiotensin-converting enzyme inhibitor ramipril. Circulation. 2001;104:1615–21. doi: 10.1161/hc3901.096700. [DOI] [PubMed] [Google Scholar]
- 16.Verdecchia P, Schillaci G, Borgioni C, et al. Prognostic significance of serial changes in left ventricular mass in essential hypertension. Circulation. 1998;97:48–54. doi: 10.1161/01.cir.97.1.48. [DOI] [PubMed] [Google Scholar]
- 17.Guide for the Care and Use of Laboratory Animals. revised 1996. NIH Publication No. 85-23 [PubMed] [Google Scholar]
- 18.Hill JA, Karimi M, Kutschke W, et al. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation. 2000;101:2863–9. doi: 10.1161/01.cir.101.24.2863. [DOI] [PubMed] [Google Scholar]
- 19.Chu V, Otero JM, Lopez O, et al. Method for non-invasively recording electrocardiograms in conscious mice. BMC Physiol. 2001;1:6–6. doi: 10.1186/1472-6793-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perucca E. Pharmacokinetic interactions with antiepileptic drugs. Clin Pharmacokinet. 1982;7:57–84. doi: 10.2165/00003088-198207010-00004. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z, Chua CC, Ho YS, et al. Overexpression of bcl-2 attenuates apoptosis and protects against myocardial i/r injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2001;280:H2313–20. doi: 10.1152/ajpheart.2001.280.5.H2313. [DOI] [PubMed] [Google Scholar]
- 23.Abbruzzese TA, Guzman RJ, Martin RL, et al. Matrix metalloproteinase inhibition limits arterial enlargements in a rodent arteriovenous fistula model. Surgery. 1998;124:328-34; discussion 334-5. [PubMed] [Google Scholar]
- 24.Hayashidani S, Tsutsui H, Ikeuchi M, et al. Targeted deletion of mmp-2 attenuates early lv rupture and late remodeling after experimental myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;285:H1229–35. doi: 10.1152/ajpheart.00207.2003. [DOI] [PubMed] [Google Scholar]
- 25.Palei ACT, Zaneti RAG, Fortuna GM, et al. Hemodynamic benefits of matrix metalloproteinase-9 inhibition by doxycycline during experimental acute pulmonary embolism. Angiology. 2005;56:611–7. doi: 10.1177/000331970505600513. [DOI] [PubMed] [Google Scholar]
- 26.Isojarvi JI, Ansakorpi H, Suominen K, et al. Interictal cardiovascular autonomic responses in patients with epilepsy. Epilepsia. 1998;39:420–6. doi: 10.1111/j.1528-1157.1998.tb01394.x. [DOI] [PubMed] [Google Scholar]
- 27.Persson H, Ericson M, Tomson T. Carbamazepine affects autonomic cardiac control in patients with newly diagnosed epilepsy. Epilepsy Res. 2003;57:69–75. doi: 10.1016/j.eplepsyres.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Tomson T, Ericson M, Ihrman C, Lindblad LE. Heart rate variability in patients with epilepsy. Epilepsy Res. 1998;30:77–83. doi: 10.1016/s0920-1211(97)00094-6. [DOI] [PubMed] [Google Scholar]
- 29.Apfelbaum JD, Caravati EM, Kerns WP, 2, Bossart PJ, Larsen G. Cardiovascular effects of carbamazepine toxicity. Ann Emerg Med. 1995;25:631–5. doi: 10.1016/s0196-0644(95)70176-1. [DOI] [PubMed] [Google Scholar]
- 30.Beutler AS, Li S, Nicol R, Walsh MJ. Carbamazepine is an inhibitor of histone deacetylases. Life Sci. 2005;76:3107–15. doi: 10.1016/j.lfs.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 31.McKinsey TA, Zhang CL, Olson EN. Signaling chromatin to make muscle. Curr Opin Cell Biol. 2002;14:763–72. doi: 10.1016/s0955-0674(02)00389-7. [DOI] [PubMed] [Google Scholar]
- 32.Kee HJ, Sohn IS, Nam KI, et al. Inhibition of histone deacetylation blocks cardiac hypertrophy induced by angiotensin ii infusion and aortic banding. Circulation. 2006;113:51–9. doi: 10.1161/CIRCULATIONAHA.105.559724. [DOI] [PubMed] [Google Scholar]
- 33.Kook H, Lepore JJ, Gitler AD, et al. Cardiac hypertrophy and histone deacetylasedependent transcriptional repression mediated by the atypical homeodomain protein hop. J Clin Invest. 2003;112:863–71. doi: 10.1172/JCI19137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Backs J, Olson EN. Control of cardiac growth by histone acetylation/deacetylation. Circ Res. 2006;98:15–24. doi: 10.1161/01.RES.0000197782.21444.8f. [DOI] [PubMed] [Google Scholar]