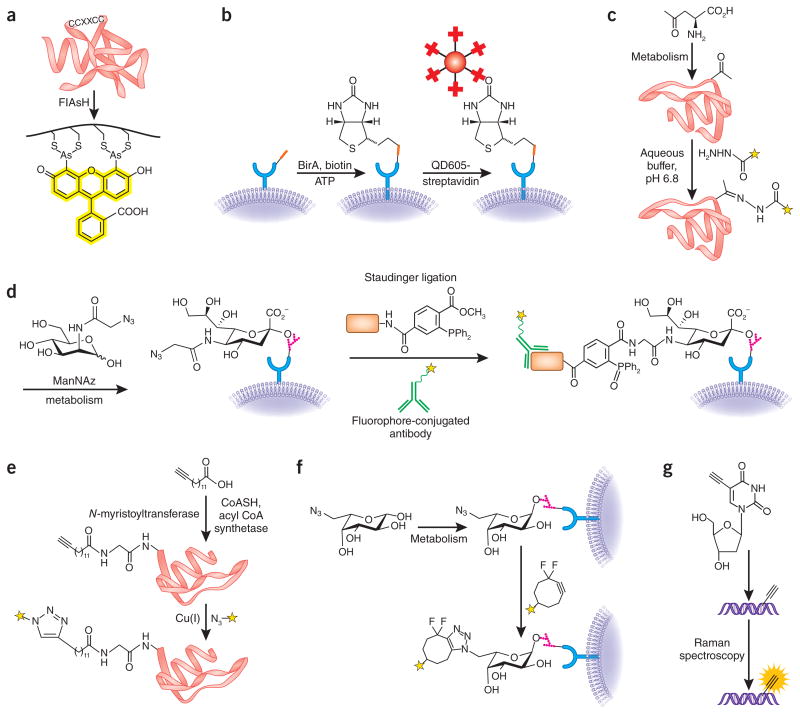
Figure 1.
Bioorthogonal reactions. (a) The biarsenical fluorescent dye FlAsH specifically reacts with target proteins genetically tagged with the tetracysteine motif4. (b) Small, genetically encoded peptide tags (red rectangle) can be specifically modified in or on live cells using engineered enzymes, such as the biotin ligase BirA6,7. Biotin-tagged target proteins can then be visualized by streptavidin-conjugated probes, such as quantum dots (QD605). (c) In an example of carbonyl condensations as a bioorthogonal method, unnatural, ketone-functionalized amino acids can be metabolically incorporated into proteins and then detected by reaction with a hydrazide-functionalized fluorescent probe (yellow star)11. (d) In an example of the Staudinger ligation, an azidosugar analog of N-acetylmannosamine (termed ManNAz) is used to metabolically label sialic acid residues on cell-surface glycoproteins. The azidosugar is detected by reaction with a tagged phosphine probe (orange rectangle) and visualized with an antibody to the tag2,21. (e) In an example of CuAAC chemistry, an alkynyl analog of myristic acid is metabolically processed to an acetyl coenzyme-A metabolite and then attached to protein N termini via N-myristoyltransferase17. The tagged protein can then be labeled via CuAAC using an azide probe (yellow star). (f) In an example of copper-free click chemistry, an azidosugar analog of fucose is metabolically incorporated into cell-surface glycans in live zebrafish embryos and detected by reaction with a fluorescent difluorinated cyclooctyne (yellow star)39,49. (g) DNA labeled with metabolically incorporated 5′-ethynyl-2′-deoxyuridine is visualized via direct Raman spectroscopic detection of the intact alkyne48.