Table 1.
Kinetic and thermodynamic parameters; pH 7.0 and 298 K
| Parameter | H2O | D2O |
|---|---|---|
| k298/s−1 | 44 ± 7 | 66 ± 10 |
| ΔH≠/kJ mol−1 | 47.5 ± 2.2 | 51.5 ± 3.7 |
| ΔS≠/J K−1 mol−1 | −56.5 ± 3.5 | −35.7 ± 2.5 |
E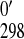 /mV /mV |
306 ± 1 | 316 ± 1 |
| ΔH0/kJ mol−1 | −46.7 ± 1.2 | −55.8 ± 2.5 |
| ΔS0/J K−1 mol−1 | −56.9 ± 1.4 | −84.4 ± 3.7 |
ΔH0 and ΔS0
are the formal enthalpy and entropy of the Cu2+/+
reduction potential, E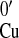 , determined as in
Eq. 3. E
, determined as in
Eq. 3. E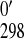 is the value at
25°C.
is the value at
25°C.
