Abstract
Intrathecal delivery of gene therapeutics is a route of administration that overcomes several of the limitations that plague current immunosuppressive treatments for autoimmune diseases of the central nervous system (CNS). Here we report intrathecal delivery of small amounts (3 μg) of plasmid DNA that codes for an immunomodulatory fusion protein, OX40-TRAIL, comprised of OX40, a tumor necrosis factor receptor, and tumor necrosis factor related apoptosis inducing ligand (TRAIL). This DNA was delivered in a formulated nucleic acid-lipid complex (lipoplex) with an asymmetric two-chain cationic lipid myristoyl (14:0) and lauroyl (12:1) rosenthal inhibitor–substituted compound (MLRI) formed from the tetraalkylammonium glycerol–based compound N-(1-(2,3-dioleoyloxy)-propyl-N-1-(2-hydroxy)ethyl)-N,N-dimethyl ammonium iodide. Delivery and expression in the CNS of OX40-TRAIL in the mouse prior to onset of experimental autoimmune encephalomyelitis (EAE), an animal model for multiple sclerosis, decreased the severity of clinical disease. We believe this preclinical demonstration of rapid, widespread, and biologically therapeutic nonviral gene delivery to the CNS is important in further development of clinical lipid-based therapeutics for CNS disorders.
Keywords: CNS gene delivery, asymmetric lipids, animal model, experimental autoimmune encephalomyelitis
Introduction
Autoimmune disorders are a unique class of diseases whose clinical complexity and unknown causes make them difficult to treat. Current treatments rely on intensive steroid regimens that produce unfavorable generalized immunosuppression. One therapeutic strategy for experimental autoimmune demyelinating disease of the central nervous system (CNS) is systemic immunogene therapy in which the therapy is delivered during disease induction.1 However, gene therapy suffers from two major problems. Many commonly used gene delivery vehicles, such as viruses, are inherently immunogenic, leading to adverse reactions and limited effectiveness. While nonviral gene therapy systems, such as biolistics, electroporation, or naked DNA administration by hydrodynamics, have reduced immunogenicity, these systems suffer from relatively low transfection efficiency, especially of postmitotic neurons or glial cells.2, 3 To attain high efficiency, large amounts of DNA were required for delivery into the CNS, joint space, or other sites of inflammation to provoke a therapeutic response. For example, intracerebral injection of 100 μg of plasmid DNA encoding transforming growth factor-β, interleukin-4, soluble dimeric human tumor necrosis factor (TNF) receptor p75, or interferon-α mixed with lipofectin was needed for a therapeutic effect in an EAE animal model.4 Peripheral routes of administration of lipoplexes, such as intramuscular injection, result in uptake of the lipoplexes in different tissues. In a mouse model of EAE, intramuscular inoculation of 50 μg of plasmid DNA (interferon-γ-inducible protein 10, a truncated diphtheria toxin, DT390) was required to penetrate the CNS and delay and alleviate disease.5
Delivery of nucleic acids (DNA, mRNA, small RNAs) is most commonly achieved by using formulated complexes with lipids. We used MLRI (Figure 1A),6,7,8 an asymmetric C(14)–C(12) cationic lipid variant of the highly active C(14)–C(14) cationic lipid dimyristooxypropyl dimethyl hydroxyethyl ammonium bromide,9 for the formation of lipoplexes because of the increased efficiency of transfection and lower toxicity of MLRI compared to that observed with symmetric lipids in mice and rats.6, 7, 10, 11 In addition, MLRI lipoplexes have been used in nonhuman primates, and the multiplex cytokine data from these experiments showed little immune response in serum or CSF to the lipoplex (Hecker J. et al, unpublished results).
Figure 1.
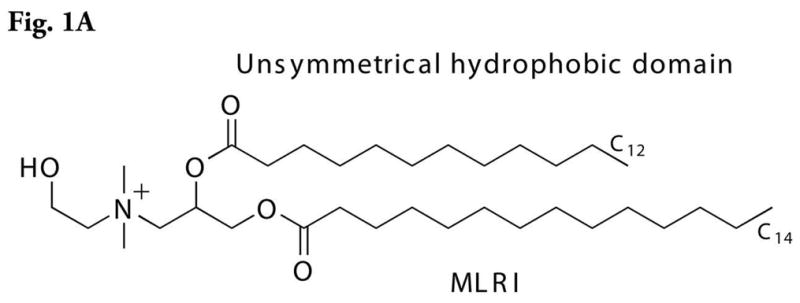
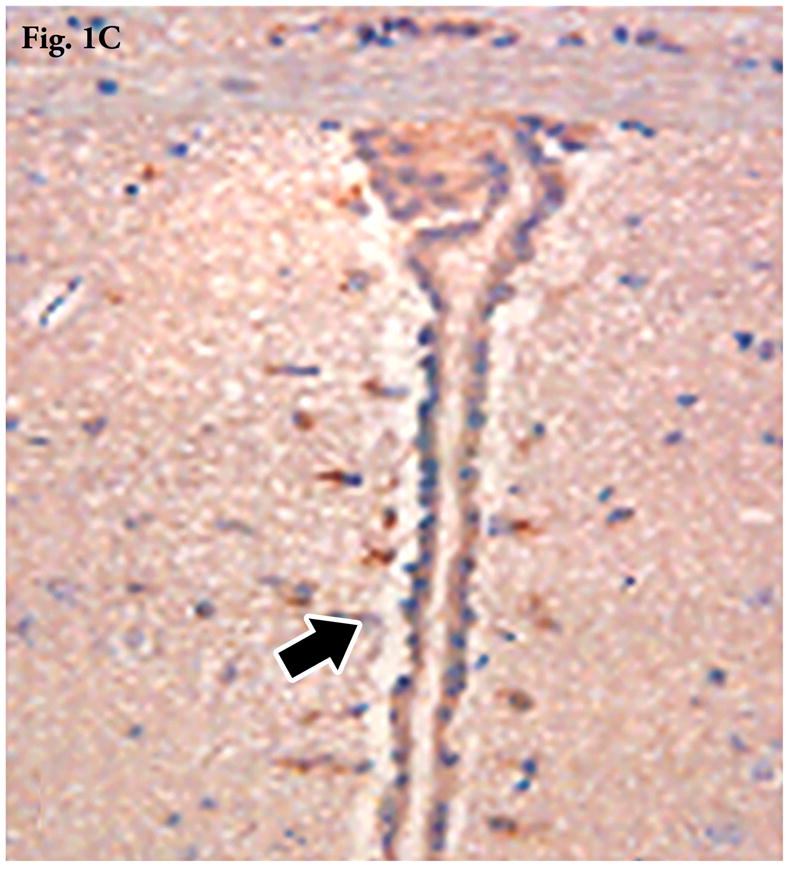
Figure 1A. Structure of MLRI.
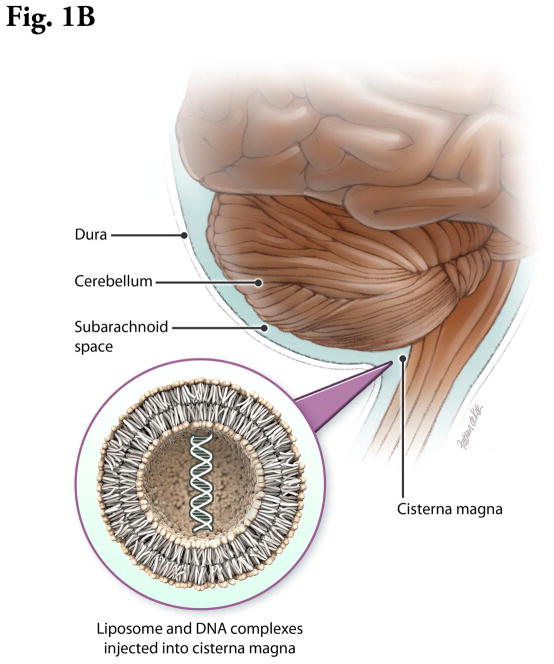
Figure 1B. Schematic of an animal model for therapeutic CNS gene delivery directly into the cerebrospinal fluid. Insert: Schematic of lipid bilayer. The actual lipoplex is a more heterogeneous structure, with condensed nucleic acid interspersed with hydrocarbon domains.
Figure 1C. Immunohistochemical demonstration of luciferase expression within the subependymal region (arrow) of the third ventricle 5 days post intrathecal injection of DNA encoding for luciferase in combination with MLRI. Brains were fixed in neutral buffered formalin, and immunohistochemistry was performed as described in materials and methods.
EAE is an animal model for multiple sclerosis caused by an autoimmune response involving pathogenic T cells directed towards the CNS.12, 13 OX40, a member of the TNF receptor super family, is found predominantly on activated encephalitogenic T cells in subjects with EAE.14, 15 OX40 ligand (OX40L)−/− mice are resistant to the induction of EAE.16 Furthermore, treatment with a monoclonal antibody to OX40L ameliorates disease.13 A fusion protein comprised of fibroblast growth factor-inducible 14 (Fn14), a receptor for blocking pro-inflammatory signaling, and TNF-related apoptosis-inducing ligand (TRAIL), a ligand capable of inhibiting pathogenic T cells, ameliorated EAE in mice.17 Based on the results from Fn14-TRAIL, another chimeric immunomodulatory protein, OX40-TRAIL, was constructed. Preliminary data indicate that OX40-TRAIL has immunomodulatory effects in a delayed-type hypersensitivity immunologic animal model (Yellayi et al., unpublished results). In the present study, mice receiving an intrathecal injection of plasmid, pOX40-TRAIL/ND (DNA encoding OX40-TRAIL cloned into the pND vector), as a lipoplex with MLRI during disease induction had decreased severity of EAE and reduced inflammatory cell infiltrates beneath the meninges compared to mice receiving pND lipoplex. Such therapeutic effects on EAE were achieved by injecting small quantities (3 μg/subject) of lipid-DNA complexes at much lower doses than previously effective doses.
Experimental Section
Luciferase DNA Vector
Construction of the expression vector for this study containing the CDNA sequence for firefly luciferase (pNDluc) from the firefly Photinus pyralis (Promega, Madison, WI) was described in previous studies.6, 8, 11 pNDluc MLRI lipoplex was administered intrathecally in five C57BL/6 mice, and brain sections were retrieved 5 days postinjection as described below.
Formulation of Lipid:DNA Complexes
Lipid-DNA complexes were evaluated previously by extensive in vitro and in vivo transfection. To obtain the maximal luciferase expression in these transfections, the charge ratio, dose, formulation time, concentration, incubation time and temperature, and lipid composition were optimized first in vitro and then in vivo.
Preparation of MLRI was described in previous studies.6, 8, 11 Briefly, chloroform was added to dry MLRI and mixed with dioleoylphosphatidyl-ethanolamine (DOPE) 50:50 in chloroform. The solution was vortexed, aliquoted into glass vials, dried, and stored at 0°C. After rehydrating the MLRI:DOPE film at 4°C, cDNA was added to the lipid preparation and incubated for 30 min at 37°C before intrathecal delivery. Stability testing revealed that the transfection ability of the rehydrated MLRI:DOPE preparation improved for several months. The lipid-DNA complexes were most active if used within 1–2 h after the start of incubation.
Induction of EAE and intrathecal gene administration
Eight-week old female C57BL/6 (n = 17) mice were immunized subcutaneously with 300 μg myelin oligodendrocyte glycoprotein (MOG) peptide fragment (amino acids 38–50) in 200 μL of phosphate-buffered saline (PBS) and incomplete Freund’s adjuvant 1:1 containing a final concentration of 2.5 mg/ml of Mycobacterium tuberculosis H37RA strain. The subcutaneous injection was divided over two injections of 100 μL each, one on either flank. Pertussis toxin (100 ng in 200 μL of PBS) was administered intraperitoneally immediately and again 48 h later to facilitate induction of disease. On Day 8 after MOG challenge, one group of mice (n = 9) received a single intrathecal injection of lipoplexes containing the plasmid vector pND, and a second group (n= 8) received lipoplexes containing pOX40-TRAIL/ND. Each type of lipoplex consisted of 3 μg of DNA in 1 μL PBS mixed with 9 μL of MLRI to achieve a final concentration of 333 ng/μL of DNA-MLRI lioplex. This 10-μL mixture was slowly injected into the cisterna magna using a Hamilton syringe.
Mice were monitored daily for 30 days postinjection and assigned a clinical score based on the following scheme: 0, no clinical signs; 1, tail weakness; 2, weak hind limbs; 3, paralyzed hind limbs; 4, weak forelimbs and paralyzed hind limbs; or 5, moribund or dead, as previously described.18 To prevent death from dehydration, mice with a score of 3 or greater received Transgel™ (Charles River, Wilmington, MA) with chow on the floor of each mouse cage. Mice without motor deficits (i.e., clinical scores of 1–2) that were able to get to food and water did not receive Transgel™. Clinical scores for each mouse in the two experimental groups were added together and then averaged to yield mean cumulative clinical scores. Statistical analysis of the clinical scores was done with the nonparametric test, Mann-Whitney test. All mice were euthanized at study end (day 30) when the clinical criteria of euthanasia (a clinical score of 4 for 3 consecutive days) was not reached.
Immunohistochemical detection of OX40 and luciferase
Spinal cord tissues were drop-fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned to a 4-μm thickness. Paraffin was removed from tissues sections on glass slides with xylene, and the sections were rehydrated in a series of alcohol washes. Heat-induced epitope retrieval was performed using citrate (pH 6.0) at 100°C for 25 min. Immunohistochemical detection of OX40 was done as described previously.19 Following intrathecal administration of pNDluc lipoplexes in five mice, immunohistochemical detection of luciferase from brain sections on day 5 postinjection was done as described previously using a polyclonal antibody against firefly luciferase (Promega, Madison, WI).20
Results and Discussion
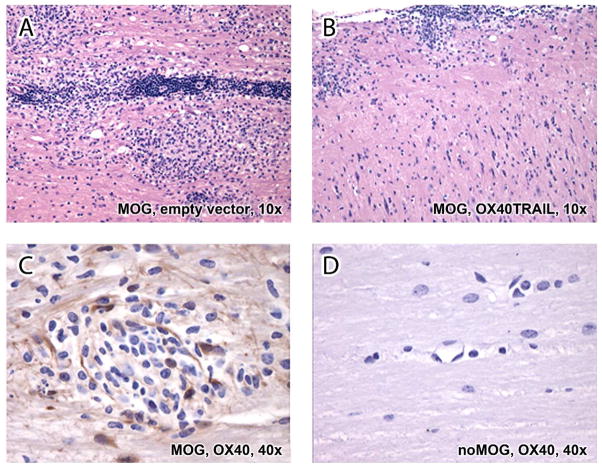
By previous sequential bioluminescent imaging, we have shown widespread distribution of transgene luciferase in the CNS for 5 or more days following intrathecal/intracranial injection of pNDluci DNA-MLRI lipoplex into the cisterna magna of mice10 and rats.8 In this study, immunohistochemical staining of brain sections on day 5 indicated luciferase expression in the cells of the ependyma as well as the subventricular zone in the mesencephalon (Figure 1C). In addition, we also investigated the expression of OX40 by immunohistochemistry in lesions in of mice with active EAE. OX40 expression was selectively increased in inflammatory cells (lymphocytes, macrophages), glial cells, and endothelial cells at inflammatory sites in the spinal cord (Figure 3C). In healthy unchallenged mice, OX40 was not expressed (Figure 3D).
Figure 3. Histopathological section of spinal cords stained with hematoxylin and eosin.
A. Following administration of empty plasmid vector pND lipoplexes and MOG, inflammatory cells composed of lymphocytes, macrophages, and occasional neutrophils infiltrated the white matter tracts, resulting in diffuse nonsuppurative myelitis.
B. Mice treated with OX40-TRAIL-MLRI and MOG had appreciably reduced number of the inflammatory cells predominantly present beneath the meninges (subpial).
C. Following MOG administration, OX40 protein was expressed by mononuclear cells, endothelial cells, and glial cells in an inflammatory site of the spinal cord.
D. Non inflamed spinal cord from an unchallenged control mouse expressed no OX40 protein.
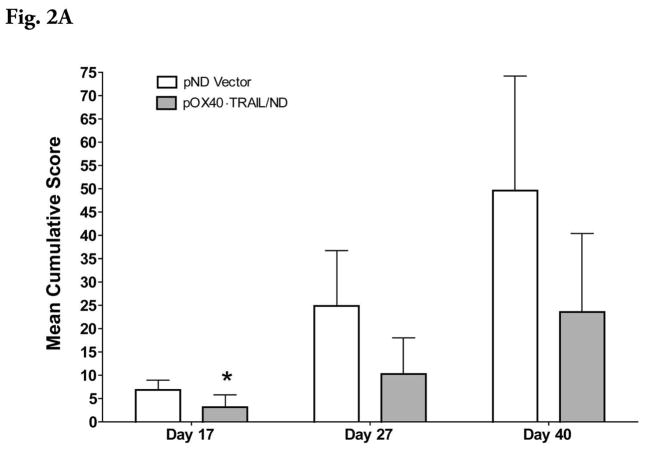
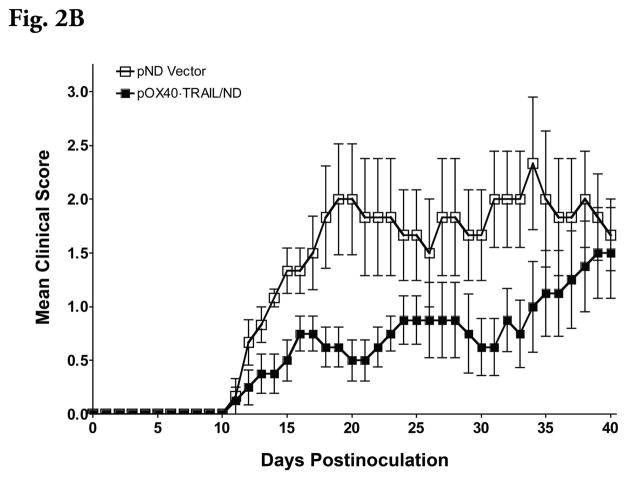
Administration of plasmid, pOX40-TRAIL/ND, as a lipoplex with MLRI into the mouse cisterna magna on day 8 after induction of EAE but prior to the onset of disease decreased the subsequent severity of the disease (Figures 2A and 2B). Mean cumulative score was significantly decreased (P ≤ 0.05) in pOX40-TRAIL/ND lipoplex-treated mice compared to that observed in pND lipoplex-treated mice at day 17 postinjection. In spinal cord tissue, inflammatory cells were appreciably reduced beneath the meninges following pOX40-TRAIL/ND ipoplex administration (Figure 3B) compared to that observed following administration of lipolexes containing the pND vector only (Figure 3A).
Figure 2.
Figure 2A. Suppression of EAE by intrathecal expression of OX40-TRAIL. One group of MOG38-50-challenged mice (n = 8) was treated with a single intrathecal injection of pOX40-TRAIL/ND lipid- complexes on day 8 postchallenge. A second group of challenged mice (n = 9) were treated with pND lipid complexes. Mice were assigned clinical scores daily. The y-axis shows the mean clinical scores for both groups of mice.
Figure 2B. Daily clinical scores were added together for each individual mouse an experimental group described in Figure 2A and then averaged to yield mean cumulative clinical scores. * significant difference between the group receiving pND empty vector lipoplexes and the group receiving pOX40·TRAIL/ND lipoplexes (P ≤ 0.05). Statistical analysis of the clinical scores was done by Mann-Whitney nonparametric test.
Injection of DNA-cationic lipid lipoplexes directly into cerebrospinal fluid facilitates fusion with the plasma membrane and uptake of DNA by astrocytes and oligodendrocytes.21, 22 Such local delivery of lipoplexes was effective in a rodent model of Parkinson’s disease.22 Injection of small quantities (10 μL of 333 ng/μL) of DNA-MLRI lipoplexes intrathecally resulted in widespread expression of the reporter protein luciferase for at least 5 days. Using the same DNA-MLRI lipoplex in healthy rat brains, a similar (7 days) duration of luciferase activity was previously observed.8
In addition, pretreatment with OX40-TRAIL DNA lipoplex prior to the onset of EAE decreased the severity of subsequent disease and infiltration of lymphocytes and macrophages to inflammatory sites. Similar to the fusion protein FN14-TRAIL, OX40-TRAIL probably acts to block OX40L actions and activate TRAIL receptor intracellular signaling pathways. Costimulatory signals by OX40L on antigen-presenting cells promote CD4+ cell clonal expansion, and the resulting cytokine responses attract activated T cells and inflammatory cells to sites of inflammation23 and exacerbate EAE.13, 15, 16, 24 Blockade of OX40L actions by the OX40-TRAIL fusion protein presumably converts proliferative OX40L signals to inhibitory TRAIL signals. TRAIL ameliorates EAE in part by down regulating the expansion of CD4+ cells (e.g., apoptosis, functional inactivation, inhibition of growth) and inhibiting cytokine responses.17, 25 In addition, TRAIL suppresses autoimmunity through the proliferation of regulatory T cells.17, 25, 26
Although the therapeutic effect, as measured by mean cumulative clinical scores, was significant for only a relatively short duration (7–10 days following disease onset), MLRI lipoplex appreciably reduced the amount of DNA needed to achieve therapeutic endpoints. Future studies will be directed toward prolonging the expression of the transgene OX40-TRAIL within the CNS and enhancing the efficiency of transfection of postmitotic neurons. The feasibility of prolonged expression of the pOX40-TRAIL/ND lipoplex depends on low toxicity. Although targeted intrathecal administration of lipoplexes bypasses some of the toxicity associated with systemic administration,27 future studies should be performed evaluating the toxicity of the pOX40-TRAIL/ND lipoplex, including immune or inflammatory responses.
In summary, we showed for the first time that widespread expression of the reporter protein luciferase for at least 5 days is possible following intrathecal injection of small quantities of pNDluc-MLRI lipoplexes. Such distribution of an expressed protein is critical for future clinical applications using vectors that deliver therapeutic genes. In addition, first time delivery of lipoplexes with transgenes for the novel recombinant fusion protein OX40-TRAIL prior to the onset of EAE significantly decreased the mean cumulative clinical scores and infiltration of cells to inflammatory sites. Further characterization of the effects of the fusion protein OX40-TRAIL on activated T cells in diseases that involve expansion of such cells may provide an increased understanding of the mechanism and utility of this fusion protein.
Acknowledgments
We thank Dr. Mark Tykocinski for providing the fusion protein (OX40-TRAIL) plasmid. We also thank Shaomin Zou and Yvette Liu for their technical assistance. This study was supported by NIH NINDS R01 NS046591 (JGH).
Abbreviations used
- CNS
central nervous system
- DNA
deoxyribonucleic acid
- DOPE
dioleoylphosphatidyl-ethanolamine
- EAE
experimental autoimmune encephalomyelitis
- Fn14
fibroblast growth factor-inducible 14 receptor
- MLRI
myristoyl (14:0) and lauroyl (12:1) rosenthal inhibitor–substituted compound formed from the tetraalkylammonium glycerol–based compound N-(1-(2,3-dioleoyloxy)-propyl-N-1-(2-hydroxy)ethyl)-N,N-dimethyl ammonium iodide
- MOG
myelin oligodendrocyte glycoprotein
- OX40L
OX40 ligand
- PBS
phosphate-buffered saline
- RNA
ribonucleic acid
- TNF
tumor necrosis factor
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
References
- 1.Piccirillo CA, Prud’homme GJ. Prevention of experimental allergic encephalomyelitis by intramuscular gene transfer with cytokine-encoding plasmid vectors. Hum Gene Ther. 1999;10(12):1915–22. doi: 10.1089/10430349950017275. [DOI] [PubMed] [Google Scholar]
- 2.Lobell A, Weissert R, Eltayeb S, Svanholm C, Olsson T, Wigzell H. Presence of CpG DNA and the local cytokine milieu determine the efficacy of suppressive DNA vaccination in experimental autoimmune encephalomyelitis. J Immunol. 1999;163(9):4754–62. [PubMed] [Google Scholar]
- 3.Baker D, Hankey DJ. Gene therapy in autoimmune, demyelinating disease of the central nervous system. Gene Ther. 2003;10(10):844–53. doi: 10.1038/sj.gt.3302025. [DOI] [PubMed] [Google Scholar]
- 4.Croxford JL, Triantaphyllopoulos K, Podhajcer OL, Feldmann M, Baker D, Chernajovsky Y. Cytokine gene therapy in experimental allergic encephalomyelitis by injection of plasmid DNA-cationic liposome complex into the central nervous system. J Immunol. 1998;160(10):5181–7. [PubMed] [Google Scholar]
- 5.Chen W, Li H, Jia Y, Lv M, Li M, Feng P, Hu H, Zhang L. In vivo administration of plasmid DNA encoding recombinant immunotoxin DT390-IP-10 attenuates experimental autoimmune encephalomyelitis. J Autoimmun. 2007;28(1):30–40. doi: 10.1016/j.jaut.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Hecker JG, Hall LL, Irion VR. Nonviral gene delivery to the lateral ventricles in rat brain: initial evidence for widespread distribution and expression in the central nervous system. Mol Ther. 2001;3(3):375–84. doi: 10.1006/mthe.2001.0272. [DOI] [PubMed] [Google Scholar]
- 7.Anderson DM, Hall LL, Ayyalapu AR, Irion VR, Nantz MH, Hecker JG. Stability of mRNA/cationic lipid lipoplexes in human and rat cerebrospinal fluid: methods and evidence for nonviral mRNA gene delivery to the central nervous system. Hum Gene Ther. 2003;14(3):191–202. doi: 10.1089/10430340360535751. [DOI] [PubMed] [Google Scholar]
- 8.Hauck ES, Zou S, Scarfo K, Nantz MH, Hecker JG. Whole animal in vivo imaging after transient, nonviral gene delivery to the rat central nervous system. Mol Ther. 2008;16(11):1857–64. doi: 10.1038/mt.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felgner JH, Kumar R, Sridhar CN, Wheeler CJ, Tsai YJ, Border R, Ramsey P, Martin M, Felgner PL. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J Biol Chem. 1994;269(4):2550–61. [PubMed] [Google Scholar]
- 10.Nantz MH, Dicus CW, Hilliard B, Yellayi S, Zou S, Hecker JG. The benefit of hydrophobic domain asymmetry on the efficacy of transfection as measured by in vivo imaging. Mol Pharm. 2010;7(3):786–94. doi: 10.1021/mp900298f. [DOI] [PubMed] [Google Scholar]
- 11.Zou S, Scarfo K, Nantz MH, Hecker JG. Lipid-mediated delivery of RNA is more efficient than delivery of DNA in non-dividing cells. Int J Pharm. 2010;389(1–2):232–43. doi: 10.1016/j.ijpharm.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsunoda I, Fujinami RS. Two models for multiple sclerosis: experimental allergic encephalomyelitis and Theiler’s murine encephalomyelitis virus. J Neuropathol Exp Neurol. 1996;55(6):673–86. doi: 10.1097/00005072-199606000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Nohara C, Akiba H, Nakajima A, Inoue A, Koh CS, Ohshima H, Yagita H, Mizuno Y, Okumura K. Amelioration of experimental autoimmune encephalomyelitis with anti-OX40 ligand monoclonal antibody: a critical role for OX40 ligand in migration, but not development, of pathogenic T cells. J Immunol. 2001;166(3):2108–15. doi: 10.4049/jimmunol.166.3.2108. [DOI] [PubMed] [Google Scholar]
- 14.Lamb LS, Jr, Abhyankar SA, Hazlett L, O’Neal W, Folk RS, Vogt S, Parrish RS, Bridges K, Henslee-Downey PJ, Gee AP. Expression of CD134 (0X-40) on T cells during the first 100 days following allogeneic bone marrow transplantation as a marker for lymphocyte activation and therapy-resistant graft-versus-host disease. Cytometry. 1999;38(5):238–43. doi: 10.1002/(sici)1097-0320(19991015)38:5<238::aid-cyto6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 15.Weinberg AD, Wegmann KW, Funatake C, Whitham RH. Blocking OX-40/OX-40 ligand interaction in vitro and in vivo leads to decreased T cell function and amelioration of experimental allergic encephalomyelitis. J Immunol. 1999;162(3):1818–26. [PubMed] [Google Scholar]
- 16.Ndhlovu LC, Ishii N, Murata K, Sato T, Sugamura K. Critical involvement of OX40 ligand signals in the T cell priming events during experimental autoimmune encephalomyelitis. J Immunol. 2001;167(5):2991–9. doi: 10.4049/jimmunol.167.5.2991. [DOI] [PubMed] [Google Scholar]
- 17.Razmara M, Hilliard B, Ziarani AK, Murali R, Yellayi S, Ghazanfar M, Chen YH, Tykocinski ML. Fn14-TRAIL, a chimeric intercellular signal exchanger, attenuates experimental autoimmune encephalomyelitis. Am J Pathol. 2009;174(2):460–74. doi: 10.2353/ajpath.2009.080462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaheer S, Wu Y, Sahu SK, Zaheer A. Suppression of neuro inflammation in experimental autoimmune encephalomyelitis by glia maturation factor antibody. Brain Res. 2011;1373:230–9. doi: 10.1016/j.brainres.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie F, Wang Q, Chen Y, Gu Y, Mao H, Zeng W, Zhang X. Costimulatory molecule OX40/OX40L expression in ductal carcinoma in situ and invasive ductal carcinoma of breast: An immunohistochemistry-based pilot study. Pathol Res Pract. 2010 doi: 10.1016/j.prp.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Deroose CM, Reumers V, Gijsbers R, Bormans G, Debyser Z, Mortelmans L, Baekelandt V. Noninvasive monitoring of long-term lentiviral vector-mediated gene expression in rodent brain with bioluminescence imaging. Mol Ther. 2006;14(3):423–31. doi: 10.1016/j.ymthe.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Ono T, Fujino Y, Tsuchiya T, Tsuda M. Plasmid DNAs directly injected into mouse brain with lipofectin can be incorporated and expressed by brain cells. Neurosci Lett. 1990;117(3):259–63. doi: 10.1016/0304-3940(90)90673-w. [DOI] [PubMed] [Google Scholar]
- 22.Cao L, Zheng ZC, Zhao YC, Jiang ZH, Liu ZG, Chen SD, Zhou CF, Liu XY. Gene therapy of Parkinson disease model rat by direct injection of plasmid DNA-lipofectin complex. Hum Gene Ther. 1995;6(11):1497–501. doi: 10.1089/hum.1995.6.11-1497. [DOI] [PubMed] [Google Scholar]
- 23.Carboni S, Aboul-Enein F, Waltzinger C, Killeen N, Lassmann H, Pena-Rossi C. CD134 plays a crucial role in the pathogenesis of EAE and is upregulated in the CNS of patients with multiple sclerosis. J Neuroimmunol. 2003;145(1–2):1–11. doi: 10.1016/j.jneuroim.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Elyaman W, Kivisakk P, Reddy J, Chitnis T, Raddassi K, Imitola J, Bradshaw E, Kuchroo VK, Yagita H, Sayegh MH, Khoury SJ. Distinct functions of autoreactive memory and effector CD4+ T cells in experimental autoimmune encephalomyelitis. Am J Pathol. 2008;173(2):411–22. doi: 10.2353/ajpath.2008.080142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda T, Hirata S, Fukushima S, Matsunaga Y, Ito T, Uchino M, Nishimura Y, Senju S. Dual effects of TRAIL in suppression of autoimmunity: the inhibition of Th1 cells and the promotion of regulatory T cells. J Immunol. 2010;185(9):5259–67. doi: 10.4049/jimmunol.0902797. [DOI] [PubMed] [Google Scholar]
- 26.Hirata S, Matsuyoshi H, Fukuma D, Kurisaki A, Uemura Y, Nishimura Y, Senju S. Involvement of regulatory T cells in the experimental autoimmune encephalomyelitis-preventive effect of dendritic cells expressing myelin oligodendrocyte glycoprotein plus TRAIL. J Immunol. 2007;178(2):918–25. doi: 10.4049/jimmunol.178.2.918. [DOI] [PubMed] [Google Scholar]
- 27.Kuo WT, Huang HY, Huang YY. Intracellular trafficking, metabolism and toxicity of current gene carriers. Curr Drug Metab. 2009;10(8):885–94. doi: 10.2174/138920009790274504. [DOI] [PubMed] [Google Scholar]