Abstract
Visual motion processing and its use for pursuit eye movement control represent a valuable model for studying the use of sensory input for action planning. In psychotic disorders, alterations of visual motion perception have been suggested to cause pursuit eye tracking deficits. We evaluated this system in functional neuroimaging studies of untreated first-episode schizophrenia (N=24), psychotic bipolar disorder patients (N=13) and healthy controls (N=20). During a passive visual motion processing task, both patient groups showed reduced activation in the posterior parietal projection fields of motion-sensitive extrastriate area V5, but not in V5 itself. This suggests reduced bottom-up transfer of visual motion information from extrastriate cortex to perceptual systems in parietal association cortex. During active pursuit, activation was enhanced in anterior intraparietal sulcus and insula in both patient groups, and in dorsolateral prefrontal cortex and dorsomedial thalamus in schizophrenia patients. This may result from increased demands on sensorimotor systems for pursuit control due to the limited availability of perceptual motion information about target speed and tracking error. Visual motion information transfer deficits to higher -level association cortex may contribute to well-established pursuit tracking abnormalities, and perhaps to a wider array of alterations in perception and action planning in psychotic disorders.
Keywords: schizophrenia, psychotic bipolar disorder, functional imaging, sensory processing, action planning, extraretinal systems
1. Introduction
The transfer of visual motion information to visual pursuit systems has been a valuable model for studying the use of sensory processing for action planning within a well-characterized neural circuitry (Lisberger, 2010). Impairments of pursuit eye movements are well-established familial abnormalities in schizophrenia (Clementz and Sweeney, 1990; Levy et al., 1993; O’Driscoll and Callahan, 2008) and have also been reported in patients with psychotic bipolar disorder (Sweeney et al., 1999; Kathmann et al., 2003; Lencer et al., 2010). Laboratory studies have suggested that altered visual motion perception may cause pursuit abnormalities in psychotic patients (Stuve et al., 1997; Sweeney et al., 1998; Chen et al., 1999; Clementz et al., 2007; Hong et al., 2009). However, little work has used functional neuroimaging to examine the implications of disturbed visual motion information processing for pursuit tracking in patients, to compare the neural substrates of these systems across different psychotic disorders, or to examine the effects of antipsychotic treatment on these systems.
Research in nonhuman primates and human functional neuroimaging studies provide a strong translational framework for clinical research of pursuit eye movement deficits (Berman et al., 1999; Lencer et al., 2004; Ilg and Thier, 2008; Kimmig et al., 2008). Extrastriate area V5 in the temporo-parieto-occipital junction represents the primary neocortical region for sensory analysis of visual motion signals, and for monitoring the discrepancy between eye and target velocity during pursuit, i.e. the retinal error signal (Newsome et al., 1985; Newsome et al., 1988; Ohlendorf et al., 2008). V5 neurons project to the intraparietal sulcus (IPS) and other parietal and temporal areas, transferring visual motion information for perceptual analysis (Ilg and Thier, 2008; Sharpe, 2008). V5 and posterior parietal areas send visual input to frontal and supplementary eye fields (FEF, SEF) for accurate visual pursuit tracking (MacAvoy et al., 1991; Rosano et al., 2002; Burke and Barnes, 2008). Dorsolateral prefrontal cortex (DLPFC) is believed to be important for velocity storage during sustained predictive pursuit, putatively due to the use of working memory resources to maintain information about target motion and performance accuracy (Lencer et al., 2004; Nagel et al., 2006; Burke and Barnes, 2008).
Some studies with functional magnetic resonance imaging (fMRI) indicate that the transfer of visual motion information to higher order brain systems is disturbed in schizophrenia (Braus et al., 2002; Martinez et al., 2008), and that V5 activation is reduced in patients while making judgments about stimulus speed and direction (Chen et al., 2008). Several psychophysical studies suggest a relationship between altered perceptual processing of visual motion and pursuit performance deficits in schizophrenia (Stuve et al., 1997; Chen et al., 1999; Kim et al., 2006; Clementz et al., 2007; Hong et al., 2009). Such a relationship might result from primary sensory deficits in V5, or from impaired information transfer from V5 to association cortex for perceptual analysis. fMRI studies using pursuit tasks have not shown V5 alterations in schizophrenia (Tregellas et al., 2005; Keedy et al., 2006) except one study that found unilateral decreased V5 activation in an exploratory analysis (Hong et al., 2005). However, we have previously demonstrated that although V5 activation was not altered during pursuit (Nagel et al., 2007), eye velocity was less linked to V5 activation in schizophrenia than in healthy subjects (Lencer et al., 2005). This would imply that V5 output may be less available to sensorimotor systems for pursuit control.
Altered transfer of visual motion information from V5 to sensorimotor systems may contribute to the reduced activation in FEF and SEF, basal ganglia, and anterior cingulate cortex that have been reported during pursuit in schizophrenia (Hong et al., 2005; Keedy et al., 2006; Nagel et al., 2007). However, as with attention demanding psychophysical tasks, there is robust top-down attentional modulation of V5 during active pursuit tracking (Corbetta et al., 1998; Chawla et al., 1999; Culham et al., 2001). Thus, independent studies of basic visual motion processing and pursuit responses are needed to evaluate bottom-up sensory processing during motion processing and the use of that information for action planning during pursuit.
The aim of the present fMRI study was to localize abnormalities during basic visual motion processing in patients with psychotic disorders, and to examine their potential relevance for the function of systems that perform sensorimotor transformation for pursuit responses. Subjects performed a paradigm requiring passive viewing of a visual motion stimulus and a second pursuit tracking paradigm. Untreated first-episode patients with schizophrenia and psychotic bipolar disorder were evaluated. To rule out the possibility that activation differences between patient groups and healthy controls during pursuit tracking might result from simple performance differences, we used a stimulus that first-episode patients have previously been shown to pursue as accurately as healthy individuals (Lencer et al., 2008; Lencer et al., 2010). We also reevaluated both patient groups after short-term treatment with a second generation antipsychotic to examine the impact of antipsychotic medication and clinical stabilization on neural networks for motion processing and pursuit.
2. Methods
2.1 Subjects
Thirty seven first-episode psychosis patients from in- and outpatient services at the University of Illinois at Chicago met DSM-IV criteria for schizophrenia spectrum disorder (N=24, Schiz) [including schizophrenia (N=21), schizoaffective disorder, depressed subtype (N=2) and schizophreniform disorder (N=1)], or bipolar I disorder with psychosis (N=13, BDP), Table 1. Diagnoses were determined at consensus conferences using all available clinical data, including results from the Structured Clinical Interview for DSM (SCID, First et al., 1995). Patients were first tested at baseline and then followed up after four weeks of treatment. At baseline, patient groups did not differ with respect to positive symptoms on the Positive and Negative Syndrome Scale (Kay et al., 1989) but negative symptoms were more pronounced in schizophrenia patients (Table 1). Twenty healthy control participants without history of Axis I disorders by SCID interview or known history of psychotic or mood disorder in first-degree relatives were recruited from the surrounding community via advertisements. There were no significant overall group differences in age or intellectual ability as assessed by the Wechsler Abbreviated Scale of Intelligence (The Psychological Corporation, 1999, Table 1).
Table 1.
Demographic and clinical characteristics of matched groupsa of first-episode patients with psychotic disorders and healthy controls
| Schizophrenia Spectrum (N = 24) | Psychotic Bipolar Disorder (N = 13) | Healthy Controls (N = 20) | |
|---|---|---|---|
| Age (SD), [Years] | 24.2 (7.5) | 23.9 (8.9) | 24.3 (4.7) |
| IQ (SD) [from WASI*] | 92.4 (18.3) | 95.2 (19.1) | 100.3 (9.6) |
| Gender (female/male) | 6/18 | 5/8 | 10/10 |
| Handedness (right/left/ambiguous) | 21/3/0 | 13/0/0 | 17/2/1 |
| Positive Symptoms score (SD) from PANSS** | |||
| Baseline | 23.5 (4.0) | 23.4 (4.1) | N/A |
| 4-week follow-up | 16.3 (4.4) | 16.9 (5.5) | |
| Negative Symptoms score (SD) from PANSS** | |||
| Baselineb | 19.3 (6.0) | 13.7 (5.8) | N/A |
| 4-week follow-up | 16.3 (6.0) | 11.4 (2.5) | |
ANOVA results for age: Fgroup(2,54) = 0.012, p=0.99, IQ: Fgroup(2,51) = 1.23, p=0.30
Differences between patient groups:
t(1,34) 7.45, p=0.01
WASI - Wechsler Abbreviated Scale of Intelligence (The Psychological Corporation 1999)
PANSS - Positive and Negative Syndrome Scale (Kay et al. 1989)
Inclusion criteria for all groups included: (1) no known systemic or neurological disease, (2) no history of head trauma with loss of consciousness > 10 minutes, (3) minimum of 20/40 far acuity, with or without correction, (4) no alcohol (24 hours), coffee, tea or cigarettes (one hour) prior to testing, and (5) no substance dependence or recent abuse according to DSM criteria except two inpatients (1 BDP, 1 Schiz) who reported cannabis abuse within four weeks of baseline studies. MR-scanning in these two patients was performed four and eight days after hospitalization, respectively. Eight of the 37 patients had been briefly treated previously in their lifetime with antipsychotic medication (1 BDP, 7 Schiz), with median cumulative lifetime exposure in previously-treated patients of 1.5 weeks (max. four weeks). For those with prior lifetime treatment, their last dose of medication was at least 60 hours prior to testing.
Fifteen schizophrenia patients, 10 psychotic bipolar patients and 15 healthy controls were available for follow-up four weeks after initial testing. Patients were treated with risperidone (N=23, 2.5mg/day (SD=1.3)), with the exception of one patient treated with olanzapine (20mg/day) and another with aripiprazole (15mg/day). One patient with schizophrenia was prescribed antidepressants at follow-up, but patients were receiving no other psychopharmacological treatment, including mood stabilizers. The follow-up sample did not differ from the original sample in demographic characteristics or baseline clinical ratings. Significant improvement after treatment was seen for positive symptoms (Ftime (1,23) = 79.61, p<0.001) without a difference between patient groups (Table 1). The study was approved by the Institutional Review Board of the University of Illinois at Chicago, and all participants provided written informed consent.
2.2 Behavioral assessment
The aim of this study was to assess visual motion processing in motion-sensitive extrastriate cortex and in parietal cortex, and relate it to activation during pursuit tracking. To achieve robust activations, especially in patients, we designed two block design paradigms during fMRI studies:
A passive motion processing task was used to assess basic responses to visual motion information. For the motion processing task (Figure 1A), a crosshair was maintained in the center of five concentric rings which gradually increased in radius (ring width 1.3°, radius of largest ring 14.5°). The Michelson Contrast of the dark rings to the background was 0.27. During activation blocks, subjects passively viewed rings moving outward at 6.7°/s while maintaining fixation of the central crosshair. Ring edges passed across the receptive fields of different retinal neurons to minimize chronic stimulation that may cause adaptation effects. During fixation blocks, the ring pattern remained static. A total of seven blocks of outward motion alternated with eight blocks of static ring pattern, each block lasting 30s.
A pursuit eye tracking task was designed in which subjects actively tracked target motion. The pursuit task (Figure 1B) used an oscillating sinusoidal waveform modified so that across the center of the display screen, between ±8°, the target moved at a constant speed of 8°/s (Lencer et al., 2008). The target was a 0.55 degree white dot and its movement across the screen covered ±12° in total. Beyond the positions of ±8°, target speed gradually decelerated to reverse its direction at ±12°. Six blocks of this pursuit task, each lasting 30s, alternated with seven 30s blocks during which subjects fixated a central white crosshair (0.55 degree). The primary performance parameter of interest for the visual tracking task was pursuit maintenance gain, defined as the ratio of eye to target speed during tracking between ±8° when target speed was at a constant speed of 8°/s (Figure 1B). Saccades were excluded from data before calculating pursuit velocity.
Figure 1.
A) Passive visual motion processing paradigm: A central crosshair was projected onto the center of five concentric rings which gradually increased in radius. During activation blocks, rings moved in outward direction at 6.7°/s while subjects maintained fixation of the crosshair. During fixation blocks, the ring pattern remained static. A total of seven blocks of outward motion alternated with eight blocks of static rings. B) Pursuit paradigm: six blocks showing an oscillating white dot for pursuit tracking alternated with seven blocks showing a central cross-hair for visual fixation (lower trace). The upper trace depicts the position signals of the target (gray) and the eye (black) during pursuit blocks. Eye velocity was analyzed in intervals between ± 8° when the target moved at a constant target speed of 8°/s (example noted in grey marked box).
Both tasks were demonstrated to the participants prior to scan sessions and were assessed within one testing session. The pursuit session was always presented first to maximize the quality of eye movement recordings. Stimuli were projected onto a rear projection screen attached to the headcoil. Eye movements were recorded during fMRI acquisition by a limbus tracker mounted on the headcoil (500 Hz, Cambridge Research Systems, UK). Measurement resolution allowed detection of saccades with a minimum amplitude of 0.5°. To calibrate the eye movement data, fixation targets were presented at 0, ± 3, 6, 9, and 12°. Data were digitized at 500Hz and visually inspected to eliminate blinks and other artifacts for further analyses.
2.3 fMRI studies
Brain imaging was performed on a 3.0 Tesla scanner using a quadrature head coil (Signa, General Electric Medical Systems, Waukesha, Wisconsin, US). fMRI studies were acquired with gradient-echo planar imaging (EPI) comprising 25 axial slices at 5mm thickness (gap 1mm; TE 25ms; TR 2000ms; flip angle 90°; matrix 64×64; voxel size 3×3×5mm; field of view 200×200mm). Anatomical images were acquired for alignment and spatial standardization. Time series data were corrected for head movement with FIASCO 5.2 (Functional Imaging Software-Computational Olio (Eddy et al., 1996). Volumes with more than 1.5 mm of motion from the median of the time series were deleted; there were no group differences in the number of volumes kept in the analyses for either time point (baseline or follow-up) or task (see supplementary material). AFNI (Analysis of Functional NeuroImages, Cox, 1996) software was used to align functional maps of individual subjects onto their anatomical images, warp each subject’s data into Talairach space, and resample functional datasets to 3×3×3mm voxels (Keedy et al., 2009). Functional activation maps (motion processing and pursuit tasks each contrasted to their fixation comparison condition) were expressed as voxel-wise Fisher’s z statistics for statistical group comparisons.
For both tasks, the first step in statistical analysis was a comparison of the two untreated patient groups and controls at baseline using a one-way ANOVA (group) and post-hoc pair-wise comparisons for brain regions where F-values in the ANOVA were significant. We used statistical procedures implemented in AFNI that evaluate both directions of group differences simultaneously. For subjects available for follow-up testing, two-way ANOVAs (group × time) were used to test for changes after treatment and clinical stabilization. Significant effects were followed up by t-tests for within group change.
Second, we related V5 activation during the passive motion processing task with whole brain activation during active pursuit to test the hypothesis that a dysfunction in processing of visual motion information from V5 was associated with atypical brain activation during visual tracking. For the purpose of correlation analyses, we extracted maximum Fisher’s z-scores in right and left hemisphere V5 (details on http://ccm.psych.uic.edu/Research/ResearchProgram/NormalBrain/ROI_rules.aspx) during the motion processing task for each participant. Since these did not differ significantly between hemispheres and there was no interaction of group by hemisphere in ANOVA, we averaged them to estimate a maximum V5 activation value for each subject (see supplementary material). The V5 maximum activation value of each subject from the motion processing task was then correlated with the individual whole brain activation during the pursuit task in each group separately in order to yield a group correlation map of V5 motion processing activation and pursuit activation. These correlation maps were then compared across groups in a pair-wise manner to reveal differences in the association of basic visual motion processing with pursuit related brain activation.
To correct for Type I error that may result from multiple comparisons or differences in samples size, the voxelwise significance threshold was set at pcorrected= 0.013 in all analyses, i.e. groups comparisons and groups correlation maps. A minimum cluster size criterion of 17 voxels (459mm3) each surpassing a puncorrected = 0.01 criterion was implemented, which was determined using AFNI’s AlphaSim Monte Carlo simulations run for in-brain only voxel space (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf).
3. Results
3.1 Behavioral data
As in prior studies (Lencer et al., 2010), there were no significant group differences for pursuit gain at baseline (Schiz: 0.89, SD=0.03, BDP: 0.90, SD=0.04, controls: 0.92, SD=0.02) or at follow-up (Schiz: 0.90, SD=0.02, BDP: 0.91, SD=0.02, controls: 0.89, SD=0.03) for the specific pursuit task used in this study. There was no subset of patients who performed substantially worse than controls as reflected in the limited performance variability within each subject group. Also, there were no significant group differences in saccade frequency before or after treatment either during the pursuit task or during the motion processing task. Thus, brain activation patterns across subject groups were compared without potential confounds related to performance differences.
3.2 Functional activation analyses
We will first present group comparisons from the motion processing task, then explain how V5 activation during motion processing was related to pursuit activation across groups and then will present group comparisons from the pursuit task.
3.2.1 Basic visual motion processing
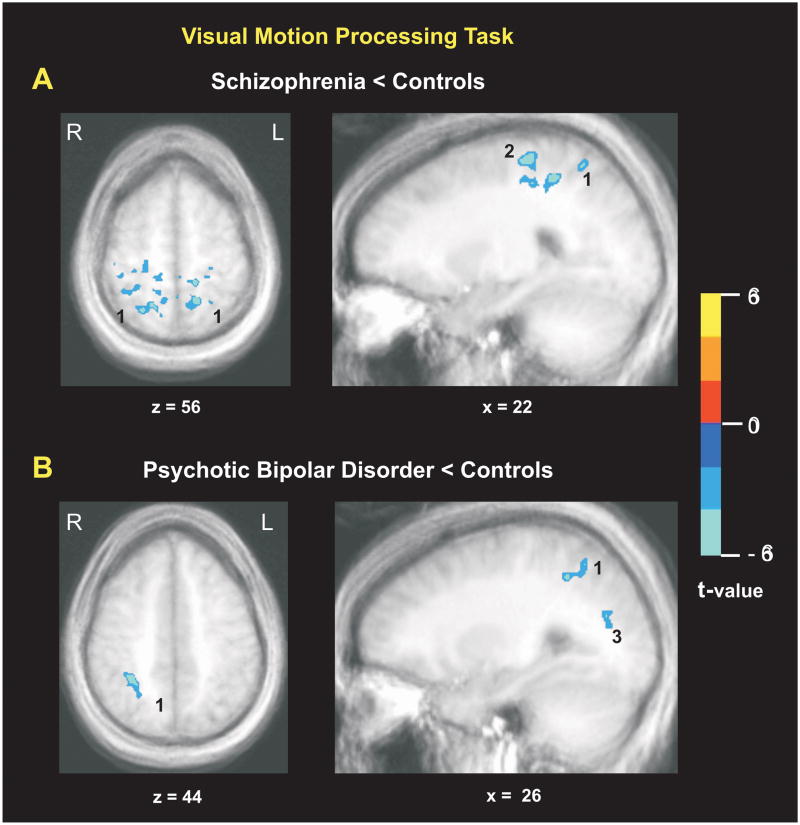
In the motion processing task, V5 activation did not differ significantly between groups., i.e. possible activation differences between groups did not surpass the contiguity cluster threshold of 17 voxels corresponding to pcorrected=0.013. Differences between groups were observed in superior parietal association cortex regions which receive robust V5 projections, and where higher level analyses of motion signals are performed. Schizophrenia patients showed significantly less activation than controls in right anterior IPS and superior parietal lobules bilaterally (Table 2, Figure 2). Psychotic bipolar patients showed reduced activation compared to controls in right superior parietal lobule, precuneus bilaterally and right V3A. The only significant difference between patient groups was a reduced activation in right posterior DLPFC in the schizophrenia group relative to the psychotic bipolar group. There were no significant group-by-time interactions, nor changes from baseline to follow-up testing in the motion processing task for any group.
Table 2.
Group differences in activation during a visual motion processing task in untreated first-episode patients with schizophrenia and psychotic bipolar disorder, and controls, pcorrected ≤0.013.
| Cortical Area | X | Y | Z | t-value at Peak Activation | Clustersize [Nvoxel] |
|---|---|---|---|---|---|
| Schizophrenia < Controls | |||||
| Anterior IPS, BA 40, right | 41 | −34 | 45 | 4.32 | 472 |
| Superior Parietal Lobule, BA 7, right | 23 | −58 | 56 | 4.08 | 45 |
| Superior Parietal Lobule, BA 7, left | −13 | −50 | 59 | 5.63 | 231 |
|
| |||||
| Psychotic Bipolar Disorder < Controls | |||||
| Superior Parietal Lobule, BA 7, right | 30 | −45 | 44 | 5.20 | 72 |
| Precuneus, right | 26 | −71 | 19 | 3.70 | 20 |
| Precuneus, left | −22 | −76 | 24 | 4.11 | 17 |
| V3A, BA 18, right | 32 | −85 | −13 | 4.57 | 22 |
|
| |||||
| Psychotic Bipolar Disorder > Schizophrenia | |||||
| Posterior DLPFC, BA 8, right | 28 | 29 | 45 | 4.56 | 19 |
BA = Brodmann Area, indicated are coordinates of peak activation
Figure 2.
Comparisons of activation during passive visual motion processing between controls (N=20) and untreated patients with (A) schizophrenia (N=24) and (B) psychotic bipolar disorder (N=13). Activations in blue show regions with less activation in patients compared to controls: 1 = superior parietal lobule, 2 = anterior intraparietal sulcus, 3 = precuneus. The color bar indicates t-values; for more detail see Table 2.
3.2.2 Association of basic visual motion processing and pursuit related activation
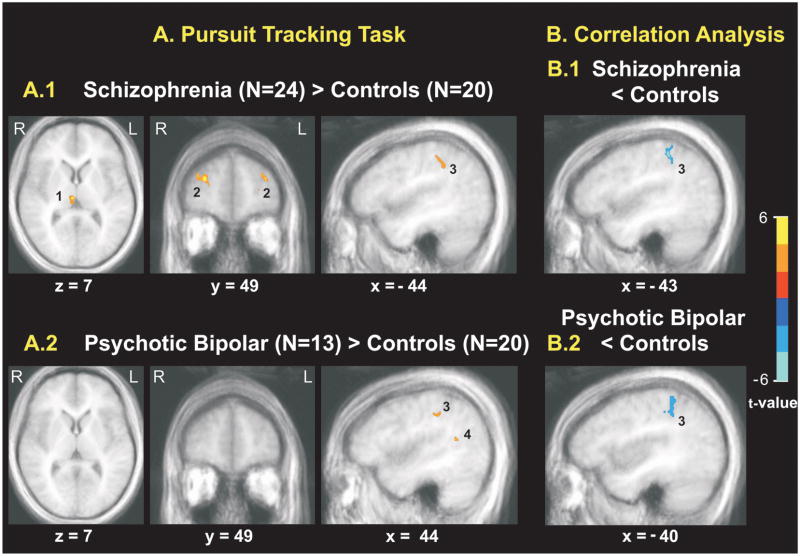
In controls, activation in V5 during the motion processing task was positively related to activation during pursuit in left V5, left anterior IPS, right superior temporal sulcus, left postcentral gyrus, left caudate, and left anterior cingulate (Table 3). In schizophrenia patients, V5 activity in the motion processing task was negatively correlated with activation during pursuit in left superior temporal sulcus, right middle temporal gyrus, parahippocampal gyrus bilaterally, left caudate and cerebellum bilaterally. The direct comparison of correlation maps between schizophrenia and control subjects revealed significantly lower correlations in schizophrenia patients in left anterior IPS, left caudate, right middle temporal gyrus, and right anterior cingulate (Table 4, Figure 3B).
Table 3.
V5 activation during passive motion processing correlated with activation across the brain during pursuit tracking in controls and untreated first-episode patients with schizophrenia, pcorrected ≤0.013
| Cortical Area | X | Y | Z | Pearson’s rat Peak Activation | Clustersize [Nvoxel] |
|---|---|---|---|---|---|
| Controls | |||||
| V5, BA 19, left | −45 | −75 | −7 | 0.79 | 20 |
| Anterior IPS, BA 40, left | −42 | −39 | 53 | 0.83 | 20 |
| Postcentral Gyrus, BA3, left | −21 | −29 | 55 | 0.78 | 26 |
| Posterior Superior Temporal Sulcus, BA 39, right | 50 | −40 | 5 | 0.79 | 20 |
| Caudate, left | −9 | 20 | 8 | 0.76 | 48 |
| Anterior Cingulate, left | −10 | 44 | 3 | 0.76 | 25 |
|
| |||||
| Schizophrenia | |||||
| Middle Temporal Gyrus, BA 21, right | 54 | −30 | −6 | −0.75 | 19 |
| Superior Temporal Sulcus, BA 41, left | −57 | −20 | 12 | −0.81 | 19 |
| Fusiform Gyrus, BA 21, left | −40 | −9 | −25 | −0.79 | 23 |
| Caudate, left | −13 | 11 | −5 | −0.80 | 17 |
| Parahippocampal Gyrus, right | 41 | −35 | −7 | −0.67 | 30 |
| Parahippocampal Gyrus, left | −32 | −25 | −16 | −0.77 | 17 |
| Anterior Cerebellum (Lobule III), left | −15 | −38 | −23 | −0.76 | 20 |
| Cerebellar Dorsal Paraflocculus (Lobule VIII), right | 24 | −54 | −46 | −0.80 | 18 |
BA = Brodmann Area; IPS = intraparietal sulcus; V5 = extrastriate area V5, indicated are coordinates of peak activation
Table 4.
Group differences in the correlation of V5 activation during a passive motion processing task with activation across the brain during visual pursuit tracking in controls and untreated first-episode patients with schizophrenia and psychotic bipolar disorder, pcorrected ≤0.013.
| Cortical Area | X | Y | Z | T-value at Peak Activation | Clustersize [Nvoxel] |
|---|---|---|---|---|---|
| Controls > Schizophrenia | |||||
| Anterior IPS, BA 40, left | −43 | −37 | 49 | 4.64 | 18 |
| Middle Temporal Gyrus, BA 21, right | 55 | −33 | −5 | 4.59 | 30 |
| Caudate, left | −15 | 12 | −6 | 5.33 | 194 |
| Anterior Cingulate, left | 6 | 25 | 11 | 3.87 | 19 |
|
| |||||
| Controls > Psychotic Bipolar Disorder | |||||
| Anterior IPS, BA 40, left | −40 | −40 | 50 | 3.35 | 29 |
| Orbitofrontal Cortex, BA 10, right | −1 | 54 | 10 | 4.50 | 21 |
|
| |||||
| Schizophrenia > Psychotic Bipolar Disorder | |||||
| Anterior Superior Temporal Gyrus, BA 38, right | 52 | −1 | −10 | 4.76 | 18 |
BA = Brodmann Area; IPS = intraparietal sulcus, indicated are coordinates of peak activation
Figure 3.
A) Comparisons of activation during pursuit tracking between controls (N=20) and untreated patients with (A.1) schizophrenia (N=24), and (A.2) psychotic bipolar disorder (N=13). Activations in red show regions with higher activations in patients compared to controls. For more detail see Table 3. B) Differences in correlations of V5 activation during motion processing with activation across the brain during pursuit tracking between controls (N=20) and untreated patients with (B.1) schizophrenia (N=24), and (B.2) psychotic bipolar disorder (N=13). Activations in blue show regions with weaker correlations in patients compared to controls. Note, that while activation in anterior intraparietal sulcus (3) was increased in patients relative to controls during pursuit its correlation with activation in visual area V5 during passive motion processing was attenuated in both patient groups implying impaired transfer of visual motion information to parietal association cortex. 1 = dorsomedial thalamus, 2 = dorsolateral prefrontal cortex, 3 = anterior intraparietal sulcus, 4 = posterior superior temporal sulcus. The color bar indicates t-values; for more detail see table 5.
In psychotic bipolar patients, V5 activation during motion processing was not correlated to activation during pursuit in any brain region. Comparison of psychotic bipolar disorder patients and controls revealed lower correlations in bipolar patients in left anterior IPS and right middle frontal gyrus (Table 4, Figure 3B). In psychotic bipolar patients, V5 activity during motion processing was less strongly correlated with activation during pursuit in right anterior superior temporal gyrus than in schizophrenia.
3.2.3 Active pursuit tracking
Schizophrenia patients had greater activation than controls in anterior DLPFC bilaterally, right posterior DLPFC, left anterior IPS, right dorsomedial thalamus, and insula bilaterally (Table 5, Figure 3A). Psychotic bipolar patients demonstrated greater activation than controls in right anterior IPS, right superior temporal sulcus and right insula. V5 activation was not altered in either patient group, nor were differences between patient groups significant. Notably, deficits were not observed in sensorimotor regions such as FEF, SEF, striatum or cerebellum on this pursuit task which patients performed as well as controls. Also, pursuit gain did not correlate with V5 activation in any group.
Table 5.
Group differences in activation during visual pursuit tracking in untreated first-episode patients with schizophrenia and psychotic bipolar disorder, and controls, pcorrected ≤0.013.
| Cortical Area | X | Y | Z | t-value at Peak Activation | Clustersize [Nvoxel] |
|---|---|---|---|---|---|
| Schizophrenia > Controls | |||||
| Anterior DLPFC, BA 9/10, right | 28 | 48 | 25 | 4.76 | 32 |
| Anterior DLPFC, BA 9/10, left | −33 | 47 | 24 | 3.5 | 30 |
| Posterior DLPFC, BA8/9, right | 45 | 26 | 35 | 3.48 | 35 |
| Anterior IPS, BA 40, left | −44 | −37 | 41 | 3.49 | 17 |
| Thalamus, right | 5 | −20 | 7 | 4.45 | 36 |
| Insula, right | 37 | −3 | 16 | 4.43 | 22 |
| Insula, left | −34 | −10 | 17 | 3.92 | 25 |
| Psychotic Bipolar Disorder > Controls | |||||
| Anterior IPS, BA 40, right | 48 | −36 | 44 | 3.25 | 17 |
| Posterior Superior Temporal Sulcus, BA 39, right | 39 | −53 | 18 | 4.09 | 26 |
| Insula, right | 37 | −2 | 17 | 5.14 | 22 |
BA = Brodmann Area, indicated are coordinates of peak activation
Exploratory analyses in the subsample available for follow-up testing revealed no significant changes from baseline during pursuit in either controls or psychotic bipolar patients. For schizophrenia patients, activation in right anterior cingulate was decreased at follow-up (x=5, y=14, z=35, tpeak= 4.01, clustersize 18 voxels).
4. Discussion
Evaluating the integrity of brain systems supporting visual motion processing and its relation to functional activation during visual pursuit of moving targets provides a strategy with particular leverage for unraveling the neural mechanisms of perceptual and action planning abnormalities in psychotic disorders. Since the sample of bipolar patients was smaller than that of both other groups, the sensitivity to detect potential patient group differences may have been limited. However, our studies of passive motion processing show similarities in the reductions of activation in target fields of V5 projections to posterior parietal cortex in both schizophrenia and bipolar patients, with no significant abnormality in motion sensitive extrastriate area V5 itself. Consistent with this, activation during the passive motion processing task was less related to pursuit activation in the anterior parietal sulcus, an area involved in extraretinal pursuit control (Heide et al., 1996; Lencer et al., 2004), in both patient groups. It is these similarities rather than differences between the patient groups that we might have been underpowered to detect that are the focus of our study. Reduced transfer of motion signals from extrastriate to association cortex could compromise pursuit generation in psychosis by impacting sensorimotor processes that depend on a precise evaluation of target motion and retinal tracking error.
For our pursuit task, we used a stimulus that does not require dynamic adjustments of pursuit velocity and acceleration as with sinusoidal tasks, or abrupt reversals in target direction as with triangular or trapezoidal waveforms. While psychotic patients have deficits on many pursuit tasks, they do not demonstrate deficits on this specific pursuit task. Thus, for the present study, activation differences are not confounded by behavioral task performance differences. During active pursuit we found that activation in prefrontal cortex, anterior intraparietal sulcus, dorsomedial thalamus and insula was greater in schizophrenia than controls. In psychotic bipolar disorder, activation during pursuit was greater than controls in anterior intraparietal sulcus, insula, and superior temporal sulcus. In the context of unimpaired performance during our pursuit task in both patient groups, these regional increases in activation may represent an increase in extraretinal drive to compensate for reduced input of visual motion information to areas in parietal cortex that support sensorimotor transformations. This would suggest that while patients are able to perform the tracking task as accurately as healthy subjects, they do so by relying on a different balance of bottom-up sensory signals and top-down cortical signals.
Restricting our sample to untreated first-episode patients diminishes potential confounds that could result from illness chronicity or medication effects. Although limited by rather small sample sizes, our follow-up post-treatment studies suggest a stability of the alterations in motion processing and pursuit systems after short-term treatment with antipsychotic medication and clinical stabilization. This observation is consistent with our previous laboratory findings that antipsychotics did not affect pursuit performance on a task similar to that used in this study (Lencer et al., 2008; Lencer et al., 2010). Reduced activation in anterior cingulate cortex seen in schizophrenia during pursuit, representing the only change at follow-up compared to baseline in any group, might reflect improved ability to allocate attention after treatment, consistent with previous observations with other tasks (Lahti et al., 2004; Abler et al., 2007; Yucel et al., 2007).
4.1 Motion processing
Human and nonhuman primate studies have demonstrated that visual motion processing, especially when stimulated by outward motion, robustly activates the magnocellular visual stream from the lateral geniculate to visual area V5, which then projects to precuneus, anterior IPS and the superior parietal lobule (Brodmann area 7, Ptito et al., 2001; Ohlendorf et al., 2008). Our finding of reduced parietal activation elicited by a simple motion stimulus is consistent with a recent EEG study on motion direction discrimination (Wang et al., 2010). However, EEG and fMRI studies using alternating gradients rather than motion stimuli have shown reduced responsivity also in primary visual cortex, which we did not observe (Butler et al., 2007; Martinez et al., 2008). This may reflect differential impairments in the response of visual cortex to gradient and motion stimuli, differential task sensitivity to occipital dysfunction, or the fact that our patients were untreated first-episode patients. Thus, while further work is needed, the present findings are consistent with a growing body of literature indicating the importance of alterations in bottom-up sensory processing in psychotic disorders. More specifically, at least as it pertains to visual motion information, the findings of the present study indicate that these alterations may result from reduced transfer of information from extrastriate cortex to heteromodal parietal projection zones, rather than from intrinsic alterations of sensory processing in V5.
Despite similar alteration in motion processing systems across bipolar disorder and schizophrenia, we also observed some differences. Reduced activation in psychotic bipolar disorder was seen in visual area V3A, which is one gateway for motion information to reach posterior parietal cortex (Orban et al., 2004). This observation suggests that motion processing may be disturbed at earlier visual processing stages in bipolar disorder than schizophrenia. Direct comparison of patient groups indicated that posterior DLPFC was more active during motion processing in bipolar than schizophrenia patients, which may reflect a greater reliance on voluntary attentional control to sustain fixation in bipolar than schizophrenia patients. This is consistent with results of recent fMRI studies using different cognitive paradigms to compare both disorders (McIntosh et al., 2008; Hamilton et al., 2009).
4.2 Association of motion processing and pursuit systems
In healthy controls, activation in V5 during passive motion processing was positively correlated with pursuit-related activation in V5 and its posterior parietal projections fields. This observation is consistent with the importance of V5 motion signals in driving pursuit responses. In both patient groups, V5 activation during motion processing had an attenuated association to activation in anterior IPS during pursuit relative to controls. This region in anterior IPS most likely corresponds to the macaque ventral intraparietal area (VIP, Bremmer, 2005) and human IPS area 5 (Konen and Kastner, 2008). Neurons in this area are believed to be crucial for using visual motion information for monitoring pursuit responses since they code target velocity during pursuit as well as they are responsive during passive viewing of optic flow patterns (Schlack et al., 2003; Konen and Kastner, 2008; Nagel et al., 2008).
Our findings suggest that sensorimotor control systems are less influenced by V5 output signals during pursuit in both patient groups. This is further supported by the observation that, in contrast to controls, V5 activation during passive motion processing was negatively rather than positively related to pursuit activations in schizophrenia patients. Such negatively correlated areas included striatal, cerebellar and temporal regions. This is in line with our interpretation that compensatory drive from extraretinal systems may increase proportionally to the level of reduced sensory input from V5 in order to sustain pursuit responses.
Association between V5 activation during motion processing and pursuit was not significantly impaired in either patient group relative to controls. This is consistent with laboratory findings implying that visual motion signals are sufficiently intact to drive a normal latency of pursuit onset and to contribute to the accurate execution of saccades to moving targets in both schizophrenia and bipolar disorder (Sweeney et al., 1998; Sweeney et al., 1999; Lencer et al., 2010). This might occur via input from V5 to FEF or cerebellum, rather than through parietal cortex.
4.3 Pursuit-related activation in schizophrenia
We did not find a pattern of consistently reduced activation in any brain region when examining pursuit-related activation in schizophrenia patients compared to controls. Rather, we observed enhanced activation in anterior and posterior DLPFC, anterior IPS, thalamus and insula relative to controls, which is consistent with the interpretation that these patients increasingly recruit extrastriate networks to optimize pursuit responses (Tregellas et al., 2004; Nagel et al., 2007). The DLPFC is involved in prediction during pursuit using working memory, i.e. velocity storage mechanisms (Schmid et al., 2001; Burke and Barnes, 2008). In healthy subjects, DLPFC activation can be up-regulated in response to reduced pursuit gain (Lencer et al., 2004; Nagel et al., 2006), and has been shown to be increased in schizophrenia during a motion judgment task (Chen et al., 2008). Therefore, greater DLPFC activation in schizophrenia patients might represent a greater utilization of top-down signals to compensate for reduced sensory input to parietal cortex. Such compensation could account for why we did not find reduced pursuit gain in patients or reduced activation in frontal lobe regions such as SEF and FEF where abnormalities have been reported with more challenging pursuit paradigms (Tregellas et al., 2004; Hong et al., 2005). This pattern of increased DLPFC activity is reminiscent of working memory findings in which schizophrenia patients perform normally with increased DLPFC activation in low demand conditions (Manoach, 2003; Quintana et al., 2003), and with similar findings of compensation in studies of other cognitive domains (Heckers et al., 1998; Bonner-Jackson et al., 2005).
Thalamic activation during pursuit (Berman et al., 1999; Tanabe et al., 2002; Nagel et al., 2008) can reflect feedback to FEF and prefrontal cortex from cerebellum about proprioceptive input from eye muscles and an efference copy of oculomotor commands (Cui et al., 2003). Therefore, increased medial thalamic activation in schizophrenia is also consistent with a greater dependence on extraretinal signals in prefrontal and parietal cortex to maintain pursuit. Increased bilateral insula activation in schizophrenia patients may represent another multimodal sensory brain area that is activated when a higher level of internal processing, motivation and action planning is needed to perform the pursuit task (Mesulam and Mufson, 1982).
4.4 Pursuit-related activation in psychotic bipolar disorder
Neuroimaging studies of pursuit in bipolar disorder have not been reported previously, although multiple laboratory studies document visual tracking deficits in this disorder (Sweeney et al., 1999; Kathmann et al., 2003; Thaker, 2008; Lencer et al., 2010). We found increased activation in anterior IPS and insula during pursuit in this patient group as in schizophrenia, suggesting an abnormality that may be common across the two psychotic disorders. Additionally, posterior superior temporal sulcus activation was increased in psychotic bipolar disorder compared to controls. This area is implicated in higher order processing relevant to attention, eye movements and motion processing in both macaques and humans (Corbetta et al., 1998; Nagel et al., 2008). Psychotic bipolar patients thus also may rely more on extraretinal processes to generate predictive pursuit, as has been shown for schizophrenia during predictive pursuit in the absence of a visual target (Nagel et al., 2007).
Direct comparison of pursuit activation between bipolar disorder and schizophrenia revealed no significant differences, failing to suggest different alterations in brain systems supporting pursuit in these two psychotic patient groups. Given considerable overlap in the form and severity of pursuit impairments in schizophrenia and psychotic bipolar disorder in laboratory studies (Lencer et al., 2010), these findings suggest a common systems-level mechanism of pursuit deficits involving reduced transfer of motion signals to parietal cortex. It remains to be determined whether the molecular/biochemical causes of these disturbances are similar as well. However, these findings parallel multiple recent reports of overlapping brain and neurobehavioral abnormalities in schizophrenia and psychotic bipolar disorder (Thaker, 2008).
The present findings document a deficit in the transfer of visual motion information from extrastriate cortex to its projection fields in posterior parietal cortex in schizophrenia and psychotic bipolar disorder. Further, the findings demonstrate an increased reliance on top-down output from extrastriate systems that may compensate for this deficit to support action planning. Even though patients behavioral performance did not differ from controls, their pursuit responses appeared to be less driven by visual sensory input to association cortex than by internally generated response plans provided by a prefrontal-parieto-thalamo network. Such a deficit might contribute to pursuit impairments in psychotic patients which have been reported with more challenging pursuit tasks than used in the present study. Alteration in the availability of visual motion information to pursuit systems represents a promising mechanistic explanation for eye tracking deficits in psychotic disorders. More generally it may provide a model for explaining how bottom-up alterations in sensory processing can contribute to perceptual abnormalities in psychotic disorders, and how top-down mechanisms diverted or utilized to compensate for this deficit.
Supplementary Material
Acknowledgments
We thank Cherise Rosen PhD, Robert Marvin MD and Peter Weiden MD from the Center for Cognitive Medicine, University of Illinois at Chicago, for their assistance in diagnostic and psychopathological assessments. This work was supported by National Institute of Health (MH62134, MH80066, MH077862 and MH083126); National Alliance for Research on Schizophrenia and Depression; Janssen (RIS-INT-35) and the Alexander von Humboldt Foundation (Feodor Lynen Fellowship to R.L. and a Humboldt Research Award to J.S).
Footnotes
Financial Disclosure: None reported
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abler B, Erk S, Walter H. Human reward system activation is modulated by a single dose of olanzapine in healthy subjects in an event-related, double-blind, placebo-controlled fMRI study. Psychopharmacology. 2007;191:823–833. doi: 10.1007/s00213-006-0690-y. [DOI] [PubMed] [Google Scholar]
- Berman RA, Colby CL, Genovese CR, Voyvodic JT, Luna B, Thulborn KR, Sweeney JA. Cortical networks subserving pursuit and saccadic eye movements in humans: an FMRI study. Human Brain Mapping. 1999;8:209–225. doi: 10.1002/(SICI)1097-0193(1999)8:4<209::AID-HBM5>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner-Jackson A, Haut K, Csernansky JG, Barch DM. The influence of encoding strategy on episodic memory and cortical activity in schizophrenia. Biological Psychiatry. 2005;58:47–55. doi: 10.1016/j.biopsych.2005.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braus DF, Weber-Fahr W, Tost H, Ruf M, Henn FA. Sensory information processing in neuroleptic-naive first-episode schizophrenic patients: a functional magnetic resonance imaging study. Archives of General Psychiatry. 2002;59:696–701. doi: 10.1001/archpsyc.59.8.696. [DOI] [PubMed] [Google Scholar]
- Bremmer F. Navigation in space--the role of the macaque ventral intraparietal area. The Journal of Physiology. 2005;566:29–35. doi: 10.1113/jphysiol.2005.082552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke MR, Barnes GR. Brain and behavior: a task-dependent eye movement study. Cerebral Cortex. 2008;18:126–135. doi: 10.1093/cercor/bhm038. [DOI] [PubMed] [Google Scholar]
- Butler PD, Martinez A, Foxe JJ, Kim D, Zemon V, Silipo G, Mahoney J, Shpaner M, Jalbrzikowski M, Javitt DC. Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain. 2007;130:417–430. doi: 10.1093/brain/awl233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla D, Buechel C, Edwards R, Howseman A, Josephs O, Ashburner J, Friston KJ. Speed-dependent responses in V5: A replication study. NeuroImage. 1999;9:508–515. doi: 10.1006/nimg.1999.0432. [DOI] [PubMed] [Google Scholar]
- Chen Y, Grossman ED, Bidwell LC, Yurgelun-Todd D, Gruber SA, Levy DL, Nakayama K, Holzman PS. Differential activation patterns of occipital and prefrontal cortices during motion processing: evidence from normal and schizophrenic brains. Cognitive, Affective & Behavioral Neuroscience. 2008;8:293–303. doi: 10.3758/cabn.8.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Levy DL, Nakayama K, Matthysse S, Palafox G, Holzman PS. Dependence of impaired eye tracking on deficient velocity discrimination in schizophrenia. Archives of General Psychiatry. 1999;56:155–161. doi: 10.1001/archpsyc.56.2.155. [DOI] [PubMed] [Google Scholar]
- Clementz BA, McDowell JE, Dobkins KR. Compromised speed discrimination among schizophrenia patients when viewing smooth pursuit targets. Schizophrenia Research. 2007;95:61–64. doi: 10.1016/j.schres.2007.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, Sweeney JA. Is eye movement dysfunction a biological marker for schizophrenia? A methodological review. Psychological Bulletin. 1990;108:77–92. doi: 10.1037/0033-2909.108.1.77. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, An International Journal. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cui DM, Yan YJ, Lynch JC. Pursuit subregion of the frontal eye field projects to the caudate nucleus in monkeys. Journal of Neurophysiology. 2003;89:2678–2684. doi: 10.1152/jn.00501.2002. [DOI] [PubMed] [Google Scholar]
- Culham JC, Cavanagh P, Kanwisher NG. Attention response functions: characterizing brain areas using fMRI activation during parametric variations of attentional load. Neuron. 2001;32:737–745. doi: 10.1016/s0896-6273(01)00499-8. [DOI] [PubMed] [Google Scholar]
- Eddy WF, Fitzgerald M, Genovese CR, Mockus A, Noll DC. Functional image analysis software — computational olio. In: Prat A, editor. Proceedings in Computational Statistics. Physica-Verlag; Heidelberg: 1996. pp. 39–49. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P) State Psychiatric Institute; New York: 1995. [Google Scholar]
- Hamilton LS, Altshuler LL, Townsend J, Bookheimer SY, Phillips OR, Fischer J, Woods RP, Mazziotta JC, Toga AW, Nuechterlein KH, Narr KL. Alterations in functional activation in euthymic bipolar disorder and schizophrenia during a working memory task. Human Brain Mapping. 2009;30:3958–3969. doi: 10.1002/hbm.20820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nature Neuroscience. 1998;1:318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Heide W, Kurzidim K, Kompf D. Deficits of smooth pursuit eye movements after frontal and parietal lesions. Brain. 1996;119 (Pt 6):1951–1969. doi: 10.1093/brain/119.6.1951. [DOI] [PubMed] [Google Scholar]
- Hong LE, Tagamets M, Avila M, Wonodi I, Holcomb H, Thaker GK. Specific motion processing pathway deficit during eye tracking in schizophrenia: a performance-matched functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:726–732. doi: 10.1016/j.biopsych.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Hong LE, Turano KA, O’Neill HB, Hao L, Wonodi I, McMahon RP, Thaker GK. Is motion perception deficit in schizophrenia a consequence of eye-tracking abnormality? Biological Psychiatry. 2009;65:1079–1085. doi: 10.1016/j.biopsych.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg UJ, Thier P. The neural basis of smooth pursuit eye movements in the rhesus monkey brain. Brain and Cognition. 2008;68:229–240. doi: 10.1016/j.bandc.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Kathmann N, Hochrein A, Uwer R, Bondy B. Deficits in gain of smooth pursuit eye movements in schizophrenia and affective disorder patients and their unaffected relatives. The American Journal of Psychiatry. 2003;160:696–702. doi: 10.1176/appi.ajp.160.4.696. [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. British Journal of Psychiatry. 1989;(Supplement):59–67. [PubMed] [Google Scholar]
- Keedy SK, Ebens CL, Keshavan MS, Sweeney JA. Functional magnetic resonance imaging studies of eye movements in first episode schizophrenia: smooth pursuit, visually guided saccades and the oculomotor delayed response task. Psychiatry Research. 2006;146:199–211. doi: 10.1016/j.pscychresns.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Keedy SK, Rosen C, Khine T, Rajarethinam R, Janicak PG, Sweeney JA. An fMRI study of visual attention and sensorimotor function before and after antipsychotic treatment in first-episode schizophrenia. Psychiatry Research. 2009;172:16–23. doi: 10.1016/j.pscychresns.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Wylie G, Pasternak R, Butler PD, Javitt DC. Magnocellular contributions to impaired motion processing in schizophrenia. Schizophrenia Research. 2006;82:1–8. doi: 10.1016/j.schres.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmig H, Ohlendorf S, Speck O, Sprenger A, Rutschmann RM, Haller S, Greenlee MW. fMRI evidence for sensorimotor transformations in human cortex during smooth pursuit eye movements. Neuropsychologia. 2008;46:2203–2213. doi: 10.1016/j.neuropsychologia.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Konen CS, Kastner S. Representation of eye movements and stimulus motion in topographically organized areas of human posterior parietal cortex. Journal of Neuroscience. 2008;28:8361–8375. doi: 10.1523/JNEUROSCI.1930-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Frey KN, Hardin M, Tamminga CA. Clozapine but not haloperidol re-establishes normal task-activated rCBF patterns in schizophrenia within the anterior cingulate cortex. Neuropsychopharmacology. 2004;29:171–178. doi: 10.1038/sj.npp.1300312. [DOI] [PubMed] [Google Scholar]
- Lencer R, Nagel M, Sprenger A, Heide W, Binkofski F. Reduced neuronal activity in the V5 complex underlies smooth-pursuit deficit in schizophrenia: evidence from an fMRI study. NeuroImage. 2005;24:1256–1259. doi: 10.1016/j.neuroimage.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Lencer R, Nagel M, Sprenger A, Zapf S, Erdmann C, Heide W, Binkofski F. Cortical mechanisms of smooth pursuit eye movements with target blanking. An fMRI study. European Journal of Neuroscience. 2004;19:1430–1436. doi: 10.1111/j.1460-9568.2004.03229.x. [DOI] [PubMed] [Google Scholar]
- Lencer R, Reilly JL, Harris MS, Sprenger A, Keshavan MS, Sweeney JA. Sensorimotor transformation deficits for smooth pursuit in first-episode affective psychoses and schizophrenia. Biological Psychiatry. 2010;67:217–223. doi: 10.1016/j.biopsych.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencer R, Sprenger A, Harris MS, Reilly JL, Keshavan MS, Sweeney JA. Effects of second-generation antipsychotic medication on smooth pursuit performance in antipsychotic-naive schizophrenia. Archives of General Psychiatry. 2008;65:1146–1154. doi: 10.1001/archpsyc.65.10.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DL, Holzman PS, Matthysse S, Mendell NR. Eye tracking dysfunction and schizophrenia: a critical perspective. Schizophrenia Bulletin. 1993;19:461–536. doi: 10.1093/schbul/19.3.461. [DOI] [PubMed] [Google Scholar]
- Lisberger SG. Visual guidance of smooth-pursuit eye movements: sensation, action, and what happens in between. Neuron. 2010;66:477–491. doi: 10.1016/j.neuron.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAvoy MG, Gottlieb JP, Bruce CJ. Smooth-pursuit eye movement representation in the primate frontal eye field. Cerebral Cortex. 1991;1:95–102. doi: 10.1093/cercor/1.1.95. [DOI] [PubMed] [Google Scholar]
- Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophrenia Research. 2003;60:285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]
- Martinez A, Hillyard SA, Dias EC, Hagler DJ, Jr, Butler PD, Guilfoyle DN, Jalbrzikowski M, Silipo G, Javitt DC. Magnocellular pathway impairment in schizophrenia: evidence from functional magnetic resonance imaging. Journal of Neuroscience. 2008;28:7492–7500. doi: 10.1523/JNEUROSCI.1852-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh AM, Whalley HC, McKirdy J, Hall J, Sussmann JE, Shankar P, Johnstone EC, Lawrie SM. Prefrontal function and activation in bipolar disorder and schizophrenia. The American Journal of Psychiatry. 2008;165:378–384. doi: 10.1176/appi.ajp.2007.07020365. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. The Journal of Comparative Neurology. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Nagel M, Sprenger A, Hohagen F, Binkofski F, Lencer R. Cortical mechanisms of retinal and extraretinal smooth pursuit eye movements to different target velocities. NeuroImage. 2008;41:483–492. doi: 10.1016/j.neuroimage.2008.02.058. [DOI] [PubMed] [Google Scholar]
- Nagel M, Sprenger A, Nitschke M, Zapf S, Heide W, Binkofski F, Lencer R. Different extraretinal neuronal mechanisms of smooth pursuit eye movements in schizophrenia: An fMRI study. NeuroImage. 2007;34:300–309. doi: 10.1016/j.neuroimage.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Nagel M, Sprenger A, Zapf S, Erdmann C, Kompf D, Heide W, Binkofski F, Lencer R. Parametric modulation of cortical activation during smooth pursuit with and without target blanking. an fMRI study. 2006;29:1319–1325. doi: 10.1016/j.neuroimage.2005.08.050. [DOI] [PubMed] [Google Scholar]
- Newsome WT, Wurtz RH, Dursteler MR, Mikami A. Deficits in visual motion processing following ibotenic acid lesions of the middle temporal visual area of the macaque monkey. NeuroImage. 1985;5:825–840. doi: 10.1523/JNEUROSCI.05-03-00825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. Journal of Neurophysiology. 1988;60:604–620. doi: 10.1152/jn.1988.60.2.604. [DOI] [PubMed] [Google Scholar]
- O’Driscoll GA, Callahan BL. Smooth pursuit in schizophrenia: a meta-analytic review of research since 1993. Brain and Cognition. 2008;68:359–370. doi: 10.1016/j.bandc.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Ohlendorf S, Sprenger A, Speck O, Haller S, Kimmig H. Optic flow stimuli in and near the visual field centre: a group FMRI study of motion sensitive regions. PloS one. 2008;3:e4043. doi: 10.1371/journal.pone.0004043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban GA, Van Essen D, Vanduffel W. Comparative mapping of higher visual areas in monkeys and humans. Trends in Cognitive Sciences. 2004;8:315–324. doi: 10.1016/j.tics.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Ptito M, Kupers R, Faubert J, Gjedde A. Cortical representation of inward and outward radial motion in man. NeuroImage. 2001;14:1409–1415. doi: 10.1006/nimg.2001.0947. [DOI] [PubMed] [Google Scholar]
- Quintana J, Wong T, Ortiz-Portillo E, Kovalik E, Davidson T, Marder SR, Mazziotta JC. Prefrontal-posterior parietal networks in schizophrenia: primary dysfunctions and secondary compensations. Biological Psychiatry. 2003;53:12–24. doi: 10.1016/s0006-3223(02)01435-x. [DOI] [PubMed] [Google Scholar]
- Rosano C, Krisky CM, Welling JS, Eddy WF, Luna B, Thulborn KR, Sweeney JA. Pursuit and saccadic eye movement subregions in human frontal eye field: a high-resolution fMRI investigation. Cerebral Cortex. 2002;12:107–115. doi: 10.1093/cercor/12.2.107. [DOI] [PubMed] [Google Scholar]
- Schlack A, Hoffmann KP, Bremmer F. Selectivity of macaque ventral intraparietal area (area VIP) for smooth pursuit eye movements. The Journal of Physiology. 2003;551:551–561. doi: 10.1113/jphysiol.2003.042994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A, Rees G, Frith C, Barnes G. An fMRI study of anticipation and learning of smooth pursuit eye movements in humans. Neuroreport. 2001;12:1409–1414. doi: 10.1097/00001756-200105250-00023. [DOI] [PubMed] [Google Scholar]
- Sharpe JA. Neurophysiology and neuroanatomy of smooth pursuit: lesion studies. Brain and Cognition. 2008;68:241–254. doi: 10.1016/j.bandc.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Stuve TA, Friedman L, Jesberger JA, Gilmore GC, Strauss ME, Meltzer HY. The relationship between smooth pursuit performance, motion perception and sustained visual attention in patients with schizophrenia and normal controls. Psychological Medicine. 1997;27:143–152. doi: 10.1017/s0033291796004230. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Luna B, Haas GL, Keshavan MS, Mann JJ, Thase ME. Pursuit tracking impairments in schizophrenia and mood disorders: step-ramp studies with unmedicated patients. Biological Psychiatry. 1999;46:671–680. doi: 10.1016/s0006-3223(99)00132-8. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Luna B, Srinivasagam NM, Keshavan MS, Schooler NR, Haas GL, Carl JR. Eye tracking abnormalities in schizophrenia: evidence for dysfunction in the frontal eye fields. Biological Psychiatry. 1998;44:698–708. doi: 10.1016/s0006-3223(98)00035-3. [DOI] [PubMed] [Google Scholar]
- Tanabe J, Tregellas J, Miller D, Ross RG, Freedman R. Brain activation during smooth-pursuit eye movements. Neuroimage. 2002;17:1315–1324. doi: 10.1006/nimg.2002.1263. [DOI] [PubMed] [Google Scholar]
- Thaker GK. Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophrenia Bulletin. 2008;34:760–773. doi: 10.1093/schbul/sbn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Psychological Corporation. Wechsler Abbreviated Scale of Intelligence (WASI) Manual. The Psychological Corporation; San Antonio: 1999. [Google Scholar]
- Tregellas JR, Tanabe JL, Martin LF, Freedman R. FMRI of response to nicotine during a smooth pursuit eye movement task in schizophrenia. The American Journal of Psychiatry. 2005;162:391–393. doi: 10.1176/appi.ajp.162.2.391. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Tanabe JL, Miller DE, Ross RG, Olincy A, Freedman R. Neurobiology of smooth pursuit eye movement deficits in schizophrenia: an fMRI study. The American Journal of Psychiatry. 2004;161:315–321. doi: 10.1176/appi.ajp.161.2.315. [DOI] [PubMed] [Google Scholar]
- Wang J, Brown R, Dobkins KR, McDowell JE, Clementz BA. Diminished parietal cortex activity associated with poor motion direction discrimination performance in schizophrenia. Cerebral Cortex. 2010;20:1749–1755. doi: 10.1093/cercor/bhp243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Brewer WJ, Harrison BJ, Fornito A, O’Keefe GJ, Olver J, Scott AM, Egan GF, Velakoulis D, McGorry PD, Pantelis C. Anterior cingulate activation in antipsychotic-naive first-episode schizophrenia. Acta Psychiatrica Scandinavica. 2007;115:155–158. doi: 10.1111/j.1600-0447.2006.00902.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.