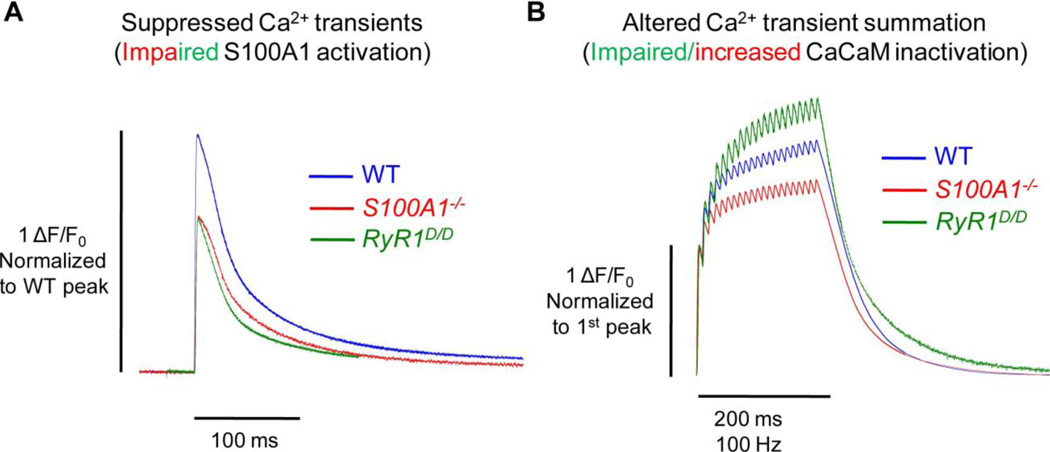
Fig. 3. S100A1 and CaM differentially regulate RyR1 Ca2+ release.
A) In response to a single action potential, the peak amplitude of the Ca2+ transient is similarly suppressed in the absence of S100A1 (S100A1−/−, red) and in fibers with a mutated CaM/S100A1 binding domain of RyR1 (RyR1D/D, green). This suggests that under resting conditions, S100A1 may predominantly occupy the CaM/S100A1 BD and potentiate Ca2+ release upon muscle activation. B) Fibers with a mutated CaM/S100A1 BD demonstrate greater relative summation of Ca2+ transients during repetitive activation. Conversely, fibers lacking S100A1 show less summation. This suggests that upon a rise in [Ca2+]i during prolonged muscle activation, Ca-CaM may predominantly occupy the CaM/S100A1 BD and inactivate some portion of Ca2+ release. This inactivation is impaired in RyR1D/D fibers, and enhanced in S100A1−/− fibers, as they lack CaM’s endogenous competitor. All data traces represent average data from [46, 53, 55].