Table 1.
Chemical structures of acridinones studied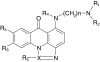
| Compound | X | n | R 1 | R 2 | R 3 | R 4 | R 5 | R 6 | ILSa | ΔT m b |
|---|---|---|---|---|---|---|---|---|---|---|
| C-1310 | C | 2 | CH2CH3 | CH2CH3 | OH | H | CH3 | H | 185 | 15.3 |
| C-1311 | C | 2 | CH2CH3 | CH2CH3 | OH | H | H | H | 93 | 13.7 |
| C-1330 | C | 2 | CH2CH3 | CH2CH3 | OCH3 | H | H | H | 96 | 11.5 |
| C-1415 | C | 2 | CH2CH3 | CH2CH3 | H | H | H | H | 55 | 7.2 |
| C-1419 | C | 2 | CH2CH3 | CH2CH3 | H | H | H | OH | 27 | 8.3 |
| C-1558 | C | 2 | CH2CH3 | CH2CH3 | C(CH3)3 | H | H | H | 0 | 2.4 |
| C-1176 | C | 2 | CH3 | CH3 | H | H | H | H | 90 | 9.5 |
| C-1263 | C | 2 | CH3 | CH3 | OH | H | H | H | 110 | 12.3 |
| C-1212 | C | 3 | CH3 | CH3 | H | H | H | H | 25 | 11.5 |
| C-1371 | C | 3 | CH3 | CH3 | OH | H | H | H | 120 | 3.5 |
| C-1554 | C | 5 | CH2CH3 | CH2CH3 | CH3 | H | H | H | 20 | 10.5 |
| C-1266 | C | 5 | CH3 | CH3 | H | H | H | H | 10 | 9.9 |
| C-1492 | C | 5 | CH3 | CH3 | OH | H | H | H | 85 | 13.1 |
| C-1233 | N | 2 | CH3 | CH3 | H | H | – | H | 77 | 9.1 |
| C-1303 | N | 2 | CH3 | CH3 | OH | H | – | H | 102 | 13.1 |
| C-1533 | N | 2 | CH3 | CH3 | OH | CH3 | – | H | 10 | 8.1 |
| C-1567 | N | 2 | CH3 | CH3 | C(CH3)3 | H | – | H | 0 | 6.8 |
| C-1410 | N | 2 | H | CH2CH3 | OH | H | – | H | 78 | 7.1 |
| C-1296 | N | 3 | CH3 | CH3 | CH3 | H | – | H | 18 | 11.5 |
| C-1305 | N | 3 | CH3 | CH3 | OH | H | – | H | 165 | 15.1 |
aThe percentage of increase in survival time of treated to control mice with P388 leukemia at optimal dose
bThe increase in DNA melting temperature (expressed in centigrade degrees) at drug to DNA base pairs 0.25 M ratio
