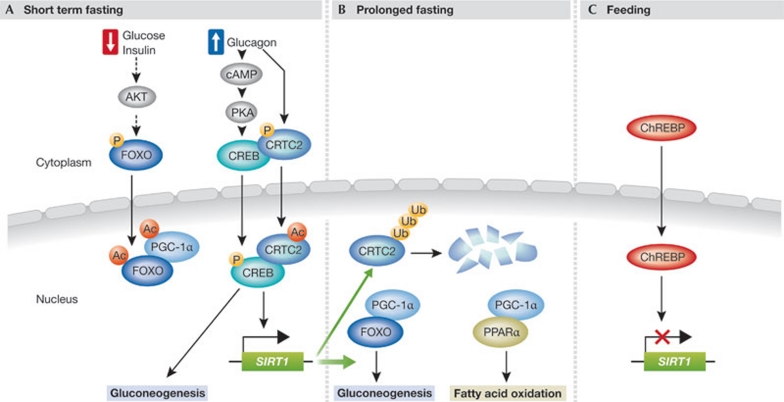
Figure 1.
Nutrient availabitily regulates SIRT1-mediated metabolic response. (A) Short-term fasting causes a decrease in blood glucose and insulin levels, and a concomitant rise in glucagon. Both of these hormonal changes initiate cellular pathways that induce gluconeogenesis. Low insulin signalling results in the dephosphorylation of the FOXO transcription factor and its translocation to the nucleus. Glucagon leads to an increase in cyclic AMP levels and activation of PKA, which phosphorylates CREB and drives its translocation to the nucleus. Glucagon also leads to the dephosphorylation of the CREB co-activator CRTC2 to trigger its translocation to the nucleus. The transcriptional complex CREB–CRTC2 activates the transcription of gluconeogenic genes and of the NAD+-dependent deacetylase SIRT1. (B) In a second phase of fasting, after a more prolonged deprivation of food, increased SIRT1 deacetylates CRTC2 and targets it for ubiquitination and degradation by the proteasome, thereby terminating the transcriptional activity of CREB. SIRT1 also deacetylates PGC-1α, FOXO and PPARα increasing their transcriptional activity. PGC-1α co-activates PPARα to induce the expression of fatty acid oxidation genes, and FOXO to maintain the expression of gluconeogenic genes. (C) In the fed state, SIRT1 expression is repressed by the activity of ChREBP transcription factor, which translocates to the nucleus. CREB, cyclic-AMP-respon¬sive-element-binding protein; CRTC2, CREB-regulated transcription coactivator 2; FOXO1, Forkhead box protein O1; PGC-1α, peroxisome proliferator-activated receptor-gamma coactivator 1α; SIRT1, sirtuin 1; ChREBP, carbohydrate responsive element-binding protein; SREBP1, sterol regulatory element binding protein 1; PPARα, peroxisome proliferator-activated receptor α. PKA, cAMP-dependent protein kinase A.