Table 1.
Molecular masses of MutS complexes as measured by native MS in 250 mM ammonium acetate pH 7.5
| MutS species | Symbol | Mass (Da) | Theoretical (Da)a |
|---|---|---|---|
| MutS monomer | |||
| MutS | 95 183 ± 12 | 95 161 | |
| MutS dimer | |||
| MutS | 190 324 ± 22 | 190 322b | |
| MutS–DNA 21-bp | 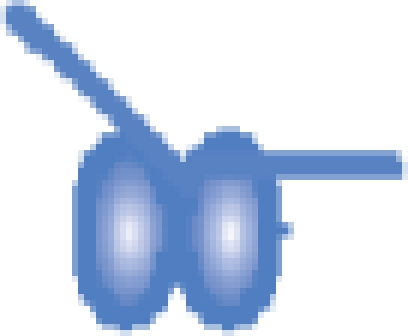 |
203 229 ± 23 | 203 191b |
| MutS–DNA 30-bp | 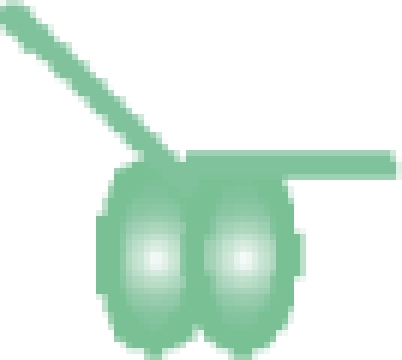 |
208 716 ± 6 | 208 753b |
| MutS tetramer | |||
| MutS |  |
381 572 ± 43 | 380 644c |
| MutS–DNA 21-bp | 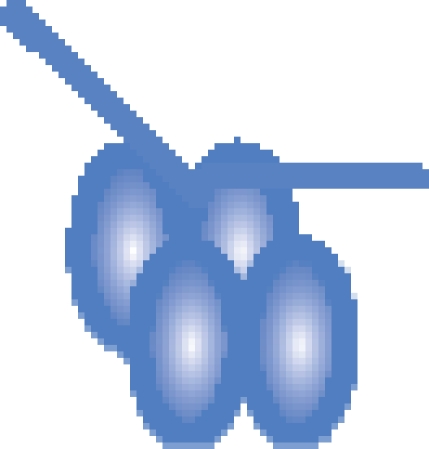 |
394 458 ± 60 | 393 513c |
| MutS–(DNA 21-bp)2 | 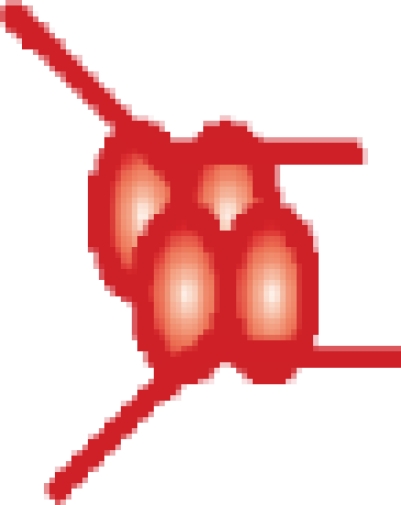 |
407 392 ± 51 | 406 380c |
Masses are determined from at least two independent measurements.
aN-terminal methionine is removed from MutS.
bThe reported masses here are only for the population of MutS dimers without nucleotide bound (that is the first peak within each dimer charge state envelope). The analysis of the masses of all three peaks, and therefore which nucleotide is bound to the two ATPase sites on the dimer, is shown in Figures 2 and 3.
c While we cannot resolve individual peaks of nucleotide binding within the MutS tetramer, the difference in mass between the observed and theoretical values (approximately 1 kDa) is in agreement with an expected average occupancy of two nucleotides per MutS tetramer.
