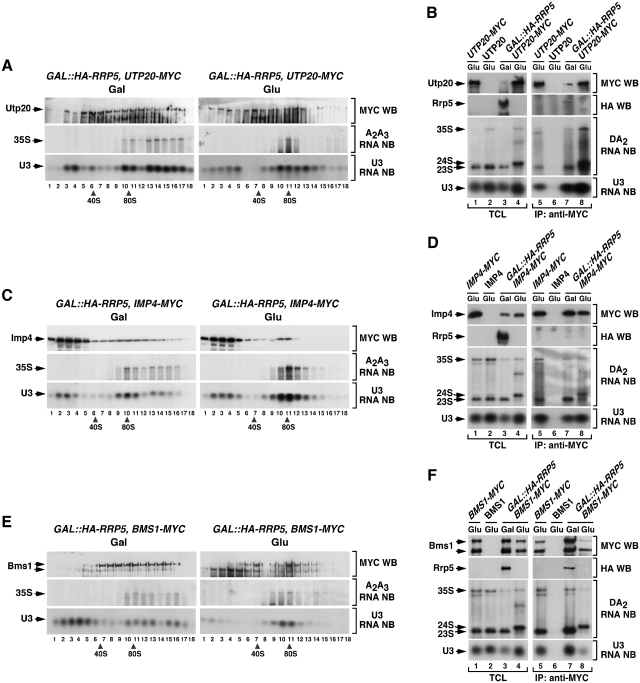
Figure 2.
The interaction of Utp20, Imp4 and Bms1 with the 35S pre-rRNA is independent of Rrp5. (A, C and E) Sedimentation behavior in sucrose gradients of Utp20 (A), Imp4 (C) and Bms1 (E) in the presence and absence of Rrp5. Cellular extracts prepared from the indicated yeast strains (top) grown in medium containing either galactose (left panels) or glucose (right panels) were resolved in 7–50% linear sucrose gradients. After ultracentrifugation, 0.5 ml fractions were collected from the top of the tube. The content of Utp20–MYC (A), Imp4–MYC (C) and Bms1–MYC (E) in each fraction was analyzed by anti-MYC immunoblotting (first panels from top). In parallel, total RNAs were prepared from each fraction and analyzed by northern blot with either an oligonucleotide probe to the 35S A2–A3 region (second panels from top) or to the U3 snoRNA (bottom panels). The number of each fraction and the sedimentation positions of 40S and 80S particles are indicated at the bottom. Gal, galactose; Glu, glucose; WB, western blot; NB, northern blot. (B, D and F) Co-immunoprecipitation of Utp20 (B), Imp4 (D) and Bms1 (F) with pre-RNAs and U3 snoRNA in the presence and absence of Rrp5. Total cellular lysates (lanes 1–4) and anti-MYC immunoprecipitates (lanes 5–8) prepared from the indicated yeast strains and growth conditions (top) were analyzed by anti-MYC and anti-HA immunoblotting (first and second panels from top), and northern blot analysis with either an oligonucleotide probe to the 35S D-A2 region (third panels from top) or to the U3 snoRNA (bottom panels). TCL, total cellular lysates; IP, immunoprecipitation.