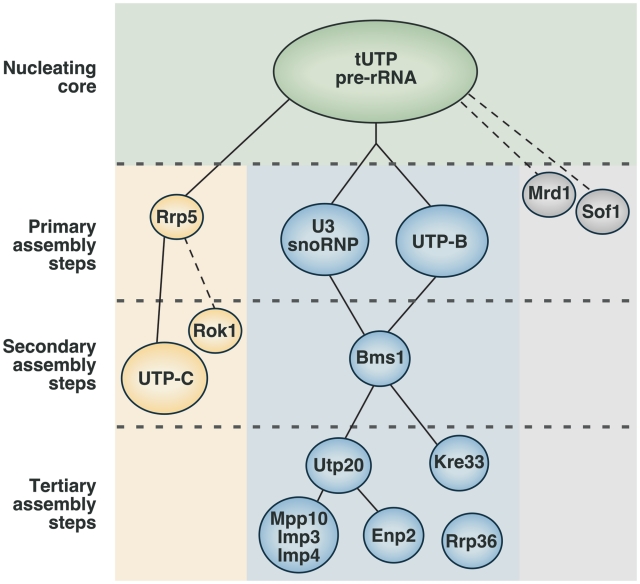
Figure 8.
Assembly hierarchy model of the 90S pre-ribosome. The binding of the tUTP subunit initiates the formation of the 90S particle and is required for the subsequent loading of the rest of the components. Two separate, and mutually independent, primary assembly steps have been identified. One of the steps involves the assembly of the U3 snoRNP and UTP-B subunits, and is required for the recruitment of at least 20 components of the particle, including the GTPase Bms1. The other primary assembly step is the binding of Rrp5, which is required for the subsequent incorporation of the UTP-C subunit. Bms1 is required for a secondary assembly step that drives the assembly of several proteins, including Utp20, Enp2, Kre33 and the Mpp10 subcomplex. This hierarchy of interactions has been established from this work and previous studies that analyzed the interdependence between factors for their binding to the 35S pre-rRNA, and the composition of pre-ribosomal complexes formed in the absence of specific proteins (18,19,25). In addition, available evidence indicates that Mrd1 and Sof1 might be primary assembly factors (37,38), and that Rok1 and Rrp36 are recruited through the Rrp5- and the UTP-B/U3-dependent branches, respectively (39,40). Rcl1, a protein that forms a subcomplex with Bms1, might be recruited to early pre-ribosomes together with Bms1. However, there is still not enough evidence to place the Rcl1–Bms1 subunit in this hierarchical model. Dashed lines indicate the possibility of intermediate assembly steps. See ‘Discussion’ section for more details about this model.