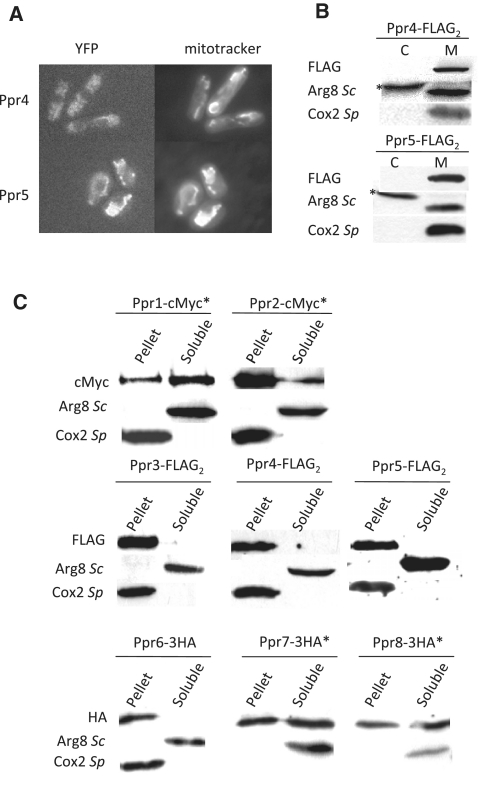
Figure 3.
Cellular localization of the S. pombe Ppr proteins. (A) Fluorescent imaging of Ppr4-YFP and Ppr5-YFP. The ppr4 and ppr5 genes were fused to YFP-Venus. Cells were grown on YPGA 2% glucose with an excess of adenine, and observed with a Zeiss Axioplan 2 microscope linked to a Cool Snap camera (Princeton Instruments). Mitotracker was obtained from Molecular Probes. The signal for Ppr4-YFP was considerably weaker than for Ppr5-YFP. As a consequence the Ppr4-YFP image also shows autofluorescence from the vacuoles. (B) Purification of mitochondria from Ppr4-FLAG2 and Ppr5-FLAG2. Cells producing FLAG-tagged Ppr4 or Ppr5 proteins were fractionated to separate the mitochondria (M) from the cytosolic fraction (C). Protein samples were subjected to western blot analysis with the anti-FLAG antibody and with two control antibodies: anti-Cox2Sp, that labels the inner mitochondrial membrane protein Cox2, and anti-Arg8Sc, that labels the soluble S. pombe mitochondrial protein Arg1 as well as a cross-reacting cytosolic protein (marked with an asterisk). An equal amount of protein was loaded in each lane. (C) Subcellular localization of all Ppr proteins. Mitochondria containing cMyc, FLAG or 3HA-tagged versions of the different Ppr proteins were alkali-treated to separate the soluble and membrane fraction, the total quantity of protein in each fraction was loaded on a 10 or 12% gel and analyzed with the appropriate anti-tag antibodies. Control antibodies are as in panel B anti-Arg8Sc for the soluble fraction and anti-Cox2Sp for the membrane fraction. The proteins marked with an asterisk were tagged at their chromosomal locus. FLAG versions were integrated at the leu1 locus in a background where the endogenous ppr gene was deleted.