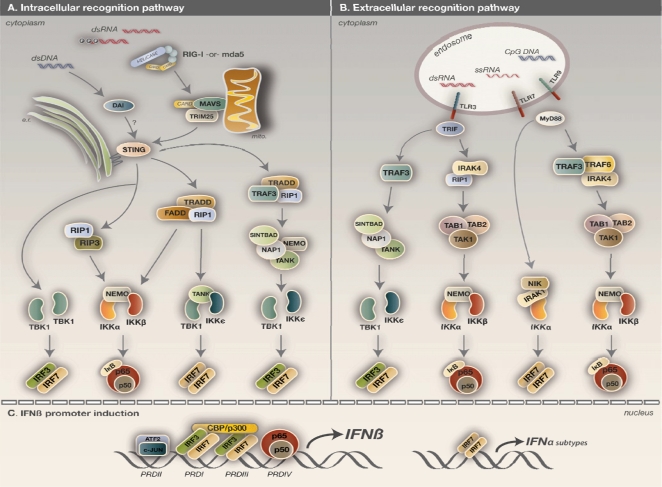
Figure 2.
Induction of type I IFN by intracellular and extracellular virus recognition pathways. A. Recognition of intracellular PAMPs is mediated by the RNA helicases, RIG-I and mda5, and DNA sensor, DAI. RIG-I and mda5 both detect distinct forms of dsRNA. RIG-I preferentially binds dsRNA containing 5′-triphosphates, whereas mda5 binds longer blunt-end dsRNA molecules. Downstream signaling via RIG-I/mda5 requires interactions with the mitochondrial-associated adaptor, MAVS, mediated by the CARD domains found on each molecule. Following ubiquitination by TRIM25 and possible association with an ER-associated factor called STING, RIG-I transmits signals downstream to distinct TRADD-containing complexes for TBK1/IKKɛ and IKKα/IKKβ activation. Activation of the IKK and IKK-related kinases results in IRF and NF-κB phosphorylation, resulting in their nuclear translocation. DAI is a recently identified PRR that detects B-form DNA found in the cytosol and induces IFN-I production through IRF3 and NF-κB activation. This mode of activation requires further investigation. However, DAI-mediated activation of IRF3 is known to require TBK1, whereas RIP1 and RIP3 are necessary for NF-κB activation. B. TLRs are expressed primarily in macrophages and DCs, and sense PAMPs found in the extracellular environment. TLR3 detects dsRNA and coordinates IRF and NF-κB activation through adaptor, TRIF. TLR7 and TLR9 sense ssRNA and CpG motifs of microbial DNA, respectively, and unlike TLR3, they utilize MyD88 to signal downstream. C. Induction of IFN-I requires the activity of the IKK and IKK-related kinases for the activation and nuclear translocation of important IRF and NF-κB TFs. The IFNβ promoter contains four PRD domains that are occupied by ATF2/c-JUN, two IRF3/IRF7 heterodimers, and p50/p65. Cooperative binding of these TFs with histone remodeling factors, CBP and p300, together form an enhanceosome that drives IFNβ production.