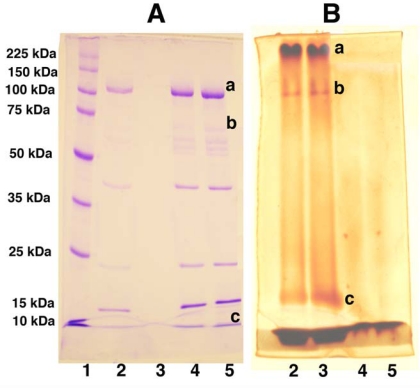
Figure 8.
Changes in the Molecular Weight of PEGylated Adenovirus Capsid Proteins Can be Detected by Gel Electrophoresis and Barium Iodide Staining. Unmodified (Lanes 4 and 5 both gels) and PEGylated (Lanes 2 and 3 both gels) adenovirus were boiled and run on 10% polyacrylamide gels with standard molecular weight markers (Lane 1 gel A) at 30 volts overnight. Duplicate samples were run such that, when electrophoresis was complete, the gel could be cut in half and either stained with Coomassie Brilliant Blue (Gel A) or a 5% barium chloride/1M iodine in 0.01 M perchloric acid (Gel B) for the identification of PEGylated proteins as described in reference 88. PEGylated proteins (Lanes 2 and 3, Gel A) are not stained as intensely as unmodified proteins (Lanes 4 and 5, Gel A) with Coomassie Blue despite the fact that the same amount of virus (based upon protein concentration) was loaded in each lane. In contrast, unmodified proteins were not resolved with barium chloride/iodide staining (Lanes 4 and 5, Gel B) while proteins with high PEG densities (Lanes 2 and 3, Gel B) could be detected by this method. Changes in the molecular weight of the adenovirus hexon (marked “a”), penton (marked “b”) and hexon-associated protein (marked “c”) were noted in the preparation included in the figure. The limit of detection of this assay was 0.5 μg of PEG in a 10% acrylamide gel.