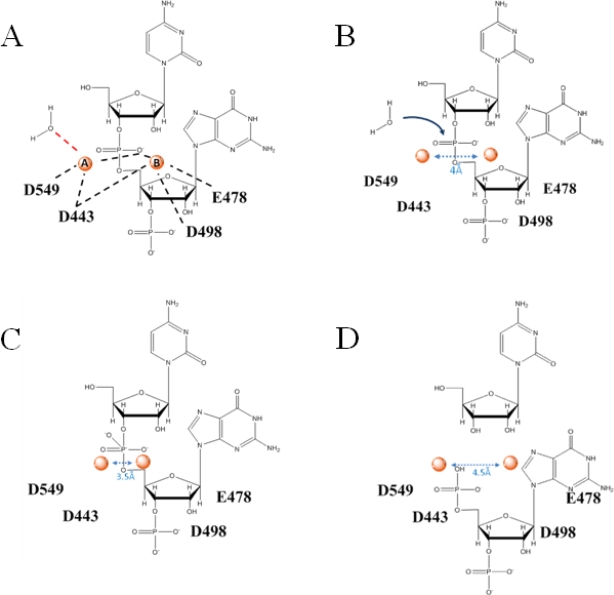
Figure 3.
The chemistry of RNase H cleavage is believed to be a two-metal ion mechanism. A Two divalent metal ions (red spheres, marked A and B) are coordinated by the active site residues D549, D443, D498 and E478 approximately 4Å apart. Metal ion A activates a water molecule. B The activated water molecule carries out a nucleophilic attack (blue arrow) driving the phosphoryl transfer reaction. C In the putative transition state, the metal ions move toward each other to bring the nucleophile within range of the scissile phosphate. D The reaction products consist of a 3′ OH group and a 5′ phosphate group, and the metal ions are again likely to be re-positioned.