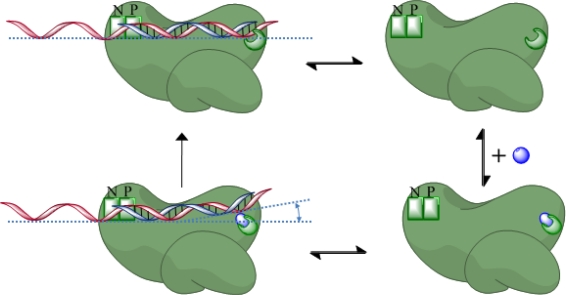
Figure 8.
A schematic of possible models of active site inhibitor binding, based on studies with the tropolone derivative β-thujaplicinol (blue sphere) [39,90]. Evidence suggests that the inhibitor is unable to bind to an enzyme-substrate (E-S) complex (top left), only to free enzyme forming (top right) and enzyme-inhibitor (E-I) complex (bottom right). However, the substrate might be able to bind to this E-I complex, forming an E-S-I complex that is not productive with respect to RNase H cleavage (bottom left). As suggested by Himmel et al., the inhibitor occupies the position normally claimed by the scissile phosphate [90]. As such, it is possible that the substrate undergoes a change in trajectory in relation to the scissile phosphate and the RNase H active site [39]. Then eventually, the inhibitor dissociates and RT is allowed to cleave the uninhibited substrate (E-S complex, top left).