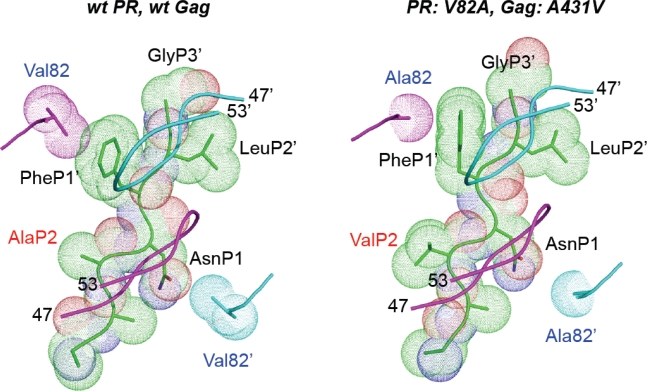
Figure 4.
Resistance-associated modification of contacts between protease and Gag. Representation of the NC/SP2 cleavage site (the amino acid backbone and surface occupancy are illustrated) within the protease active site. Left: wild-type protease and Gag. Right: protease carrying a V82A resistance mutation and Gag harboring the A431V mutation. Both views are from above the flap region of protease (top views). The backbones of the flaps (residues 47 to 53) are illustrated as a light blue- and a violet ribbon above the surface of the cleavage site. The protease residues (Val and Ala for wild type and mutated protease, respectively) at position 82 and 82’ in the active site of the enzyme are shown. Replacement of Val82’ by the smaller Ala82’ in the resistant protease (bottom right of the right panel) results in the loss of contact with the Asn residue in position P1 (AsnP1) of the cleavage site. This can be partly compensated by the Gag mutation A431V, which creates a protrusion in position P2 of the cleavage site (ValP2 on the right panel, in red). This is expected to create a new contact with the protease active site (not shown), at a position that is not normally occupied by the substrate.