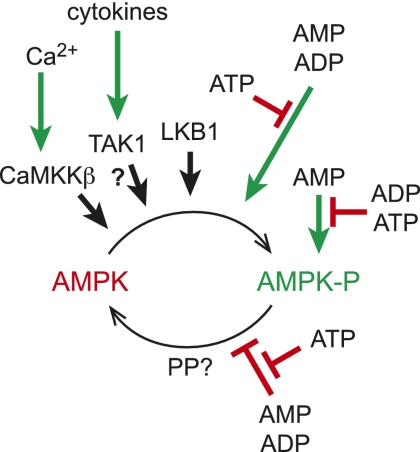
Figure 1.
Regulation of AMPK by phosphorylation and adenine nucleotides. AMPK is activated >200-fold by phosphorylation at Thr 172, catalyzed by three upstream kinases: (1) LKB1, which appears to be constitutively active; (2) TAK1, which is activated by cytokines (as the physiological significance of this remains uncertain, it is shown with a question mark); and (3) CaMKKβ, which is activated by a rise in cytosol Ca2+. Phosphorylation is stimulated and dephosphorylation is inhibited by conformational changes triggered by binding of AMP or ADP to the γ subunit of AMPK, both effects being antagonized by ATP. AMP binding also causes a further 10-fold allosteric activation; this effect is antagonized by both ATP and ADP. The identity of the protein phosphatase that dephosphorylates Thr 172 (PP?) remains unclear.