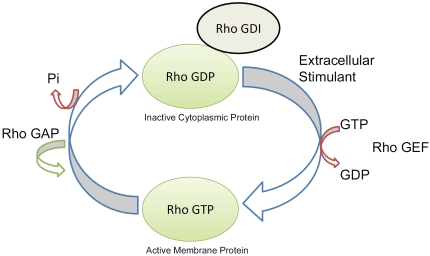
Figure 3.
Rho GTPases switch between an active GTP-bound and an inactive GDP-bound state modulated by a family of regulators. Inactive Rho-GDP forms a complex with the GDP-dissociation inhibitor (Rho-GDI) in the cytoplasm and remains GDP-bound unless activated by a stimulant and by the guanine nucleotide exchange factors (RhoGEF) which exchange GTP for GTP. Rho in its GTP-bound form is then translocated to the membrane, where it acts on its target protein as a GTPase. Then, GTPase activating proteins (RhoGAP) enhance GTPase activity and reconvert activated Rho to its inactivated GDP bound form, after which, it is translocated to the cytoplasm by Rho-GDI.