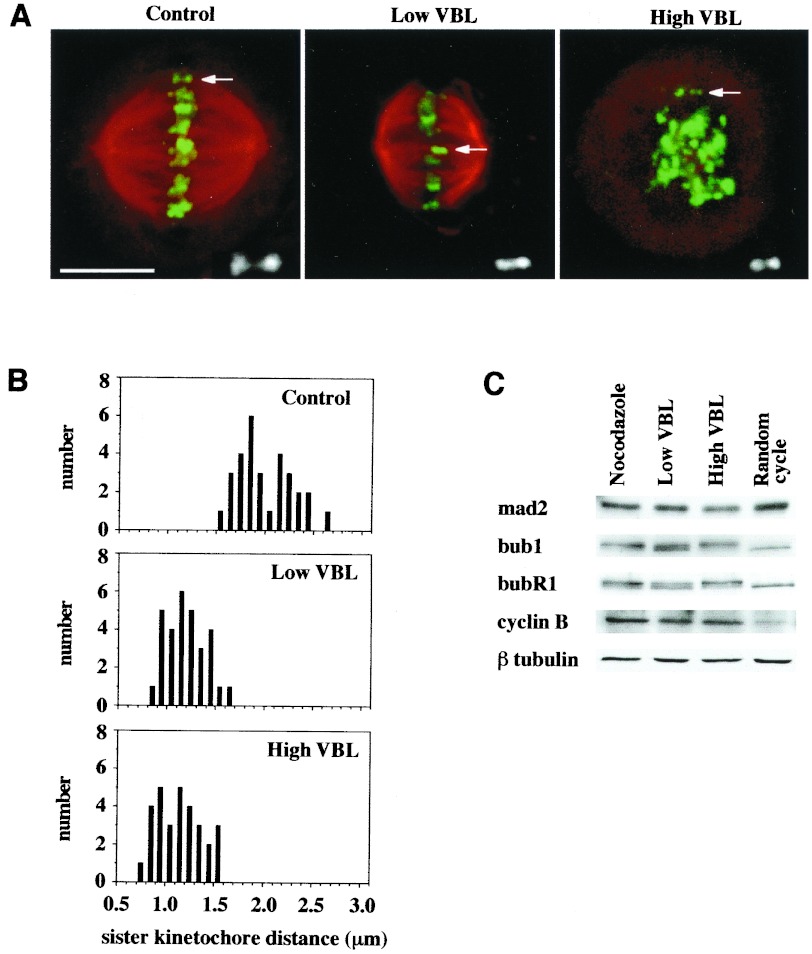
Figure 1.
Treatment of HeLa cells with low concentrations of vinblastine suppresses microtubule dynamics while maintaining a bipolar mitotic spindle and attached kinetochores. (A) Double-label immunofluorescence microscopy for centromeres (green; detected with CREST variant scleroderma human autoimmune serum) and antitubulin (red) demonstrates that 6.7 nM VBL does not perturb bipolar spindle association with kinetochores, as compared with untreated control metaphase cells. By contrast, 0.5 μM VBL induces complete disassembly of the mitotic spindle. (Bar = 10 μm.) Insets show enlargements of kinetochore pairs representative of those used for measurements. Point-to-point measurements were made by using comos software. Kinetochore pairs were selected by their close proximity, presence in the same focal plane, and, when at metaphase, alignment with the long axis of the spindle. Measured distances of the Inset kinetochores shown were: control, 2.04 μm; L-VBL, 1.24 μm; H-VBL, 1.16 μm. (B) VBL (6.7 nM) suppresses spindle tension at kinetochores, reducing the distance between sister kinetochores relative to that of untreated control metaphases (from 1.95 ± 0.28 μm to 1.15 ± 0.2 μm), yielding values comparable to those in 0.5 μM VBL-treated cells with no spindles (1.1 ± 0.23 μm). (C) The whole cell levels of mad2, bub1, and bubR1, as determined by immunoblotting, remain constant in mitotic cells in which spindle dynamics are suppressed (6.7 nM VBL) or in which the spindle is disassembled (0.5 μM VBL or 1.7 μM nocodazole). Cyclin B blots are shown to confirm mitotic status. β-tubulin blots are shown to confirm equal loading of all extracts.