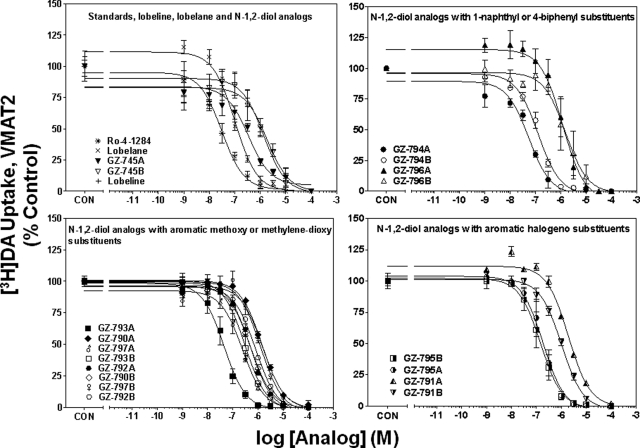
Fig. 5.
N-1,2-Diol analogs inhibit [3H]DA uptake into vesicles prepared from rat striatum. For clarity of presentation, compounds are grouped according to structural similarity of additions to the phenyl rings: standards, lobeline, lobelane, and N-1,2-diol analogs (top left), N-1,2-diol analogs containing 1-naphthyl or 4-biphenyl substituents (top right), N-1,2-diol analogs containing aromatic methoxy or methylenedioxy substituents (bottom left), or N-1,2-diol analogs containing aromatic halogeno substituents (bottom right). Nonspecific [3H]DA uptake was determined in the presence of 10 μM Ro-4-1284. Control (CON) represents specific vesicular [3H]DA uptake in the absence of analog (34.1 ± 1.18 pmol/mg/min). Symbol inset shows compounds in order from highest to lowest affinity. n = 4 rats/analog.