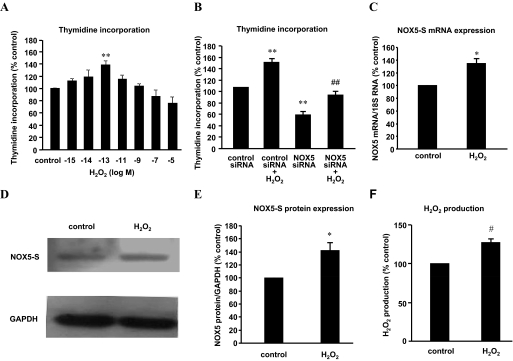
Fig. 1.
H2O2 up-regulates NOX5-S expression in FLO cells. A, FLO cells were incubated with different concentrations of H2O2 (10−5, 10−7, 10−9, 10−11, 10−13, 10−14, 10−15 M) for 48 h and then incubated with methyl-[3H]thymidine (0.05 μCi/ml) for 4 h. H2O2 (10−13 M) significantly increased thymidine incorporation, whereas higher doses (10−5 and 10−7 M) slightly decreased thymidine incorporation. B, FLO cells were treated with H2O2 (10−13 M, 48 h) 24 h after NOX5 siRNA and control siRNA were introduced into FLO cells by Lipofectamine 2000. Knockdown of NOX5-S significantly decreased thymidine incorporation at basal condition and in response to H2O2 treatment. C, FLO cells were treated with 10−13 M H2O2 for 48 h, and then NOX5-S mRNA levels were measured by real-time PCR. H2O2 (10−13 M) significantly increased NOX5-S mRNA levels. D and E, a typical image of three Western blot analyses (D) and summarized data (E) show that 10−13 M H2O2 significantly increased NOX5-S protein level. FLO cells were treated with 10−13 M H2O2 for 48 h. F, FLO cells were treated with 10−13 M H2O2 for 48 h, washed, and cultured for an additional 24 h. H2O2 levels in culture medium were measured by using an Amplex Red H2O2 assay kit. A 48-h H2O2 treatment significantly increased H2O2 production (n = 3). t test, *, P < 0.05, #, P < 0.01; ANOVA, **, P < 0.01, compared with control or control siRNA group; ANOVA, ##, P < 0.01, compared with control siRNA plus H2O2 group.